Section contents:
Angiosperms (flowering plants)
- Flowers
- Life cycle
- Pollination
- Fruits
- Fruit & seed dispersal ←
- Leaf architecture
- Overview of angiosperm phylogeny
Feature image. A selection of fruits showing structural modifications for different modes of dispersal. Left: Uncarina ankaranensis fruit showing barbs for adherence to animal fur (epizoochory). Center: Box elder (Acer negundo) with winged fruits for wind dispersal (anemochory). Right: Bladdernut (Staphylea colchica) with inflated capsules that may facilitate water dispersal (hydrochory); two individual seeds also shown. Credits: Acer negundo (MHNT.BOT.2007.40.12) and Staphylea cochica (MHNT.BOT.2006.70.4) by Roger Culos and Uncarina ankaranensis (MHNT.BOT.2011.18.22) by Didier Descouens (all from Muséum de Toulous, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Introduction
Seed dispersal—the movement of a seed away from its parent plant, often facilitated by a vector (e.g., animals, wind)—has several potential advantages. On the level of the individual, dispersal provides an opportunity for seedlings to establish themselves away from their parent plants, potentially occupying new and/or more favorable habitats. Dispersal also facilitates more genetic mixing in a population because related individuals are less likely to be clustered close to one another.
The unit of dispersal in angiosperms may be the seed itself, or a seed (or seeds) enclosed within a fruit. Fruits or seeds of angiosperms are often modified to enhance dispersal. Dispersal may occur by a number of different means, including gravity (basically, a simple means of dispersal involving the seed falling and potentially rolling downslope a short distance), wind, water, animals, and ballistic dispersal (adaptations that launch seeds from the fruit). Dispersal syndromes are suites of fruit or seed traits that correlate with certain modes of dispersal. For example, wings are associated with wind-dispersal, whereas fleshy structures are associated with animal dispersal. As with pollination syndromes, dispersal syndromes can be used to infer the likely dispersal mode of a particular fruit or seed type. It should be noted, however, that mode of dispersal may differ from—or may be more variable than suggested by—the structural attributes of a particular type of fruit or seed. Units of dispersal (in this case, fruits or seeds that serve as the units of dispersal) are called diaspores or disseminules.
The earliest angiosperms typically had small disseminules that did not exhibit many specialized modifications to facilitate dispersal. During the Paleogene, fruit and seed size became more diverse, and fossilized disseminules commonly exhibit specialized adaptations to enhance dispersal; most notably, fleshy fruits (animal-dispersed), nuts (animal-dispersed), and winged fruits and seeds (wind-dispersed) became diverse and abundant. Fruits and seeds with hairs (wind-dispersed) and spines (often animal-dispersed by adherence) are present but less common in the Paleogene fossil record (discussed here). In fossil angiosperms, dispersal mode is typically inferred from the structural attributes of disseminules (fruits or seeds) and/or the dispersal modes of modern taxa related fossil plants.

Fossil fruits with different adaptations for dispersal. Left: Endocarp (fruit stone or pit) of Suciacarpa xiangae (Late Cretaceous, Spray Formation, Vancouver Island, Canada), possibly adapted for dispersal by ingestion; the fleshy structure that likely surrounded the endocarp is not preserved. Scale bar = 2 mm. Center: Nuts (Corylus johnsonii, Eocene, Klondike Mountain Formation, Washington, U.S.A.), probable dispersal by storage/caching. Right: Winged fruits (Dillhoffia cachensis, Eocene, Klondike Mountain Formation, Washington, U.S.A.), probable dispersal by wind. Credits: Suciacarpa xiangae, Holotype, SH 790, Fig. 1A in Atkinson et al. (2017) Int. J. Plant Sci. (CC BY 4.0); Corylus johnsonii, SR 99-07-10 (Kevmin, via Wikimedia Commons, CC BY-SA 3.0); Dillhoffia cachensis, SR 92-17-20 (Kevmin, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Animal dispersal (zoochory)
Many animal-dispersed fruits are dispersed by vertebrates—especially certain mammals and birds, although fish and reptiles can also act as dispersal agents—or ants. Vertebrate-dispersed fruits and seeds may be fleshy, or may have fleshy coverings; ant-dispersed seeds often have nutrient-rich appendages.
Dispersal by ingestion (endozoochory)
Many of the fleshy fruits that humans enjoy—such as raspberries (Rubus) and cherries (Prunus)—are adapted for dispersal by vertebrates. Some fleshy fruits are consumed with seeds intact. The seeds pass through the digestive tract of an animal and are deposited elsewhere; germination may be enhanced by weakening of the seed coat as it passes through the digestive tract. Alternatively, seeds may be covered by a hard inner fruit wall (the endocarp, also known as the pit or stone) that is not digested. Sometimes, seeds may be regurgitated rather than passing entirely through the digestive tract. Dispersal through transport in the gut of an animal is endozoochory (Greek endon + zooin = within animal).
In the fossil record, seeds that were probably dispersed via ingestion are often found without the surrounding fleshy fruiting structure, so dispersal by ingestion must often be inferred by comparison to modern plants. Occasionally, however, more direct evidence may be discovered. For example, well-preserved fruits may retain fleshy structures. Seeds may also be found in coprolites (fossilized poop), demonstrating that they were ingested and passed through an animal's digestive tract (see here for one study). Seeds may even be found within a fossilized animal's gut (see here, for example).

Ancient and modern feces showing evidence of seed dispersal. Left: Modern American black bear (Ursus americanus) feces with partially digested fruits and seeds or pits. Right: Moa (Moa) coprolite (fossilized feces) from New Zealand with coprosma (Coprosma) seeds. Credits: Black bear scat (Courtney Celley, USFWS Midwest Region, via flickr, CC BY 2.0); moa coprolite (Jo Carpenter, via The Conversation, CC BY-SA 4.0). Images modified from originals.
In addition to fleshy fruits and fruit appendages, seeds themselves can have fleshy structures that attract animals. In such cases, the seed and its fleshy structure or covering may be consumed whole. In other cases (like ant-dispersed seeds with elaiosomes, discussed below), the fleshy structure alone is consumed, with the seed left behind. Examples of fleshy seed structures are:
- Aril: An aril is a fleshy or leathery structure that partially or completely surrounds a seed. An example is the red aril that surrounds the nutmeg (Myristica fragrans) seed and yields the spice mace.
- Sarcotesta: A sarcotesta (from the Greek sarx, flesh) is a fleshy layer of the seed coat. Sarcotestae are perhaps best known from the seeds of some gymnosperms (e.g., the stinky sarcotesta on Ginkgo seeds), but can also be found on seeds of some angiosperms like magnolia (Magnolia) and pomegranate (Punica granatum).
As with fleshy fruits, direct evidence for these fleshy seed structures or appendages may be hard to come by in the fossil record, so comparison to extant relatives may suggest the mode of dispersal.
Dispersal by ants (myrmecochory)
Seed dispersal by ants is known as myrmecochory (Gree, myrmēx = ant). Seeds dispersed by ants often have small fat- and protein-rich appendages called elaiosomes. Ants typically transport seeds with elaiosomes to their nests, where they detach the elaiosomes from the seeds; thus, ant dispersal tends to occur over short distances. Ant dispersal, as inferred by the presence of elaiosomes on seeds, is widespread in flowering plants; a study estimated that ant dispersal has evolved more that 100 times in angiosperms (see here).
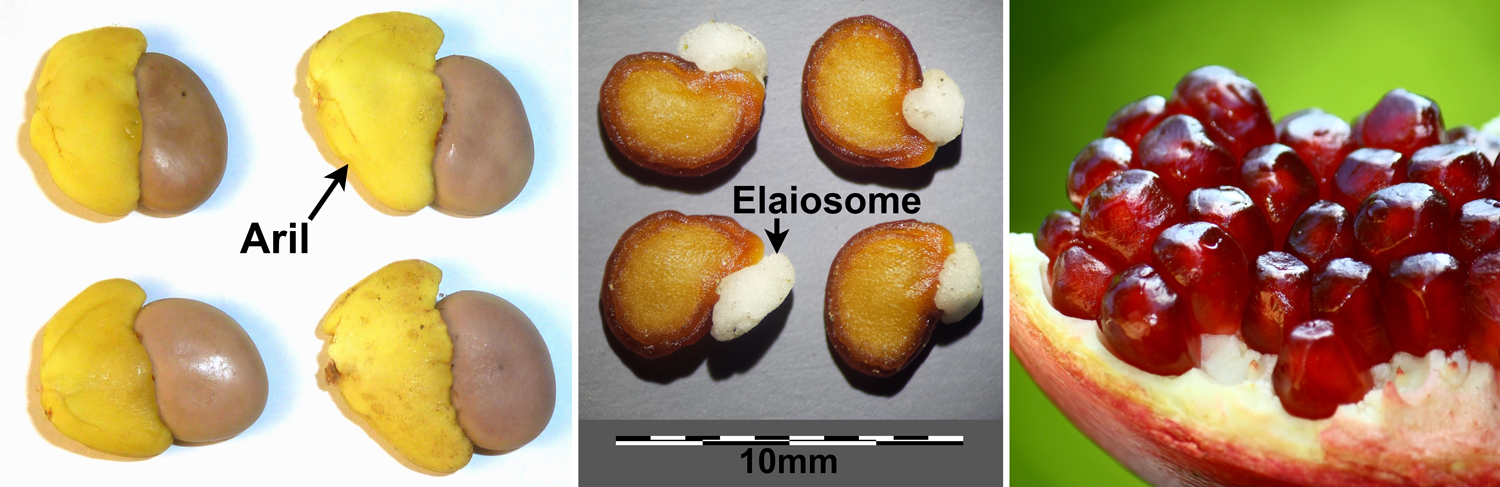
Fleshy/food structures on seeds. Left: Arils on seeds of weeping boer-bean (Schotia brachypetala). Center: Elaiosomes on seeds of prickly burr (Datura innoxia). Right: Pomegranate (Punica granatum) seeds, each with a fleshy sarcotesta. Credits: Schotia brachypetala seeds (JMK, via Wikimedia Commons, CC BY-SA 3.0); Datura innoxia seeds (Stefan.Iefnaer, via Wikimedia Commons, CC BY-SA 4.0); Punica granatum (Anton Croos/Art of Photography blog, via Wikimedia Commons, CC BY-SA 4.0). Images modified from originals.
Dispersal by caching or hoarding
The embryos and stored food within seeds themselves are often attractive to vertebrate dispersers. Caching or hoarding animals, like squirrels and some types of birds (such as jays), gather and cache (store) seeds and/or dry fruits in order to eat them later. Although many fruits and seeds may thus be consumed, some will be ignored or forgotten, providing them the opportunity to grow into new plants. Fruits of hickories, walnuts, and oaks, for example, are dispersed by seed-eating animals. In the fossil record, we may find feeding traces on fruits or seeds. Occasionally, food caches may also be identified.

Animal fruit/seed hoarders. Some animals collect and store fruits or seeds as a future food source; this behavior may help disperse seeds. Left: An eastern gray squirrel (Sciurus carolinensis) with a walnut (Juglans); squirrels cache nuts and revisit the caches later. Right: An acorn woodpecker (Melanerpes formicivorus) with an acorn (Quercus). Acorn woodpeckers store acorns in granaries, which are made up of a series of holes drilled in a live or dead trees. Credits: Black squirrel (Grendelkhan, via Wikimedia Commons, CC BY-SA 3.0); acorn woodpecker (Mike's Birds, via Wikimedia Commons, CC BY-SA 2.0). Images modified from originals.
Dispersal by adherence (epizoochory)
Epizoochory (Greek epi + zoion = on animal) is dispersal by adherence to the outside of an animal's body. A common method by which fruits are distributed in this way is to adhere to the fur or feet of a mammal. Fruits adapted for adherence are often covered with hook-like structures, sometimes minute and sometimes large and imposing. It should be noted, however, that spines or hooks may occur in water-dispersed fruits or may be present as defensive structures rather than as structures to aid dispersal.
Dispersal by adherence. The fruits above are dispersed by becoming caught in animal fur or on or in the feet of animals. Left: Fruits of greater burdock (Arctium lappa); the hooked appendages on burdock were the inspiration for the development of velcro. Right: Fruit of grapple plant (Harpagophytum procumbens). Credits: Arctium lappa MHNT.BOT.2004.0.16 and Harpagophytum procumbens MNHT.BOT.2005.0.1243 (Roger Culos, Muséum de Toulouse, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Wind dispersal (anemochory)
Fruits and seeds that are wind-dispersed frequently have modifications that help slow their descent to the ground and increase the chances that they will be blown laterally by air currents, so that they do not land directly beneath or next to their parent plant. Seed modifications for wind dispersal can include small size and/or light weight, wings, hairs, and/or inflation.
One of the most obvious modifications for wind-dispersal is the wing. Winged fruits are common in the fossil record beginning in the Paleogene. Winged fruits or seeds often have a single wing, in which case the wing may be asymmetrical, or offset to one side of the fruit or seed. If they have more than one wing, the wings many be regularly arranged around the fruit or seed. Often, the structure of the wing or wings will cause a seed or fruit to spin or rotate as it falls (known as autorotation, i.e., self-rotation). Maples (Acer) produce familiar wind-dispersed fruits that spin as they fall. If you live in a neighborhood with maple trees, you can observe this yourself; watch the mature fruits as they fall from a tree on a windy day, or pick up the fallen mericarps (fruit halves) and drop them to watch them spin as they fall.

Winged fruits. Left: Winged mericarps (indehiscent parts of a fruit, each developing from one carpel and containing one seed) of amur maple (Acer ginnala); these fruits will rotate while falling. Middle: Winged fruit of Cedrelospermum, an extinct member of the elm family (Eocene, Florissant Formation, Colorado, U.S.A.); the position of the wing probably caused this fruit to rotate. Right: Multi-winged fruit of Chaneya (Eocene, Florissant Formation, Colorado, U.S.A.). Credits: Acer ginnala mericarps (Steve Hurst, hosted by USDA-NRCS PLANTS Database, no copyright); Cedrelospermum and Chaneya (Florissant Fossil Beds National Monument, National Park Service, no copyright). Images modified from originals.
The Javan cucumber (Alsomitra macrocarpa) produces lightweight seeds that are able to glide for long distances on large wings that resemble hang-gliders. Other winged fruits and seeds may rock back and forth as they fall, or follow a spiral path while falling. Lightweight disseminules may have hairs that act as parachutes, allowing them to drift on air currents. Examples include the familiar fruits of the dandelion (Taraxacum), plane tree (Platanus), and cattail (Typhus), as well as seeds of milkweed (Asclepias) and cottonwood (Populus).

Gliders and parachutes. Left: Winged, gliding seed of Javan cucumber (Alsomitra macrocarpa). Right: Achenes of American sycamore (Platanus occidentalis) with tufts of hairs that aid in wind dispersal. Credits: Alsomitra macrocarpa seed (Scott Zona, via Wikimedia Commons, CC BY 2.0); Plantanus occidentalis (Steve Hurst, hosted by the USDA-NRCS PLANTS Database, no copyright). Images modified from originals.
Parachuting dandelion fruits. Video showing how the pappus on the fruit (not seed!) of the dandelion (Taraxacum) helps it float on air currents. Credit: The secret physics of dandelion seeds (Nature Video, via YouTube).
Inflated fruits may also be dispersed by wind. The papery inflated fruits of golden raintree (Koelreuteria, shown below) are often considered to be wind-dispersed, whereas similar fruits of bladdernut (Staphylea, shown at the top of this page) are often said to be water-dispersed. In reality, it is possible that these inflated fruits have more than one mode of dispersal, which could also be determined by the habitat that the parent tree is occupying. (See also the groundcherry/Physalis fruits described below.)

Inflated capsules of koelreuteria/rain tree (Koelreuteria). Left. Capsules of golden raintree (Koelreuteria paniculata). Right. Capsule of Koelreuteria allenii (Eocene, Florissant Formation, Colorado, U.S.A.). Credits. Koelreuteria fruits (E.J. Hermsen, DEAL); Golden-rain tree fruit (Florissant Fossil Beds National Monument, National Park Service, public domain). Images modified from originals.
Water dispersal (hydrochory)
Plants that live in wetland environments or near the ocean may have buoyant, or floating, fruits or seeds. Cranberries (some species of Vaccinium) are low-growing plants found in boggy environments. Their bright red berries are not particularly sweet, and thus probably not terribly attractive to animals. Cranberries do, however, float, which aids in their dispersal in wetland habitats. It has been hypothesized that cranberries evolved from ancestors that had more palatable, animal-dispersed fruits. Humans take advantage of the berries' buoyancy during commercial production, as cranberry bogs can be flooded so that the floating berries can be more easily collected.
Some plants with floating fruits or seeds can disperse long distances over the ocean. The most obvious example of this is the coconut palm (Cocos nucifera), which has large, fibrous fruits that can float to and colonize oceanic islands. Similarly, legumes in the genus Entada produce large, buoyant seeds; each seed harbors an air pocket, which enhances it ability to float.

Buoyant fruits and seeds. Left: Workers harvesting cranberries (Vaccinium) fruits, which are naturally buoyant. Right: Seeds of box bean (Entada phaseoloides), a legume with buoyant seeds; note the air space in the seed that has been cut open. Credits: Cranberry harvest in New Jersey (Keith Weller, USDA-ARS, via Wikimedia Commons, Public Domain); Entada phaseoloides MHNT seeds (Didier Descouens, Muséum de Toulouse, via Wikimedia Commons, CC BY-SA 4.0).
Buoyancy cannot generally be tested directly in fossil fruits, so water-dispersal of fossil fruits may be inferred based on plant relationships, ecology, and structural comparison to fruits of modern plants. An interesting example is provided by fossil groundcherry (Physalis) fruits that were discovered in Eocene lake sediments of the Laguna del Hunco flora of South America. Groundcherries produce fleshy berries surrounded by an inflated calyx that looks like a paper lantern. The scientists who described the fossil groundcherries speculated that they may have been water-dispersed, and observed that modern groundcherries surrounded by intact lanterns can float (see here). A later experimental study confirmed that the lanterns surrounding modern groundcherries could plausibly aid in their dispersal by water, but noted that they could also aid in wind-dispersal (see here). Since the fruits are also fleshy, groundcherry seeds can potentially be dispersed in three ways: ingestion, water, and wind.

Groundcherry (Physalis) fruits. Left: Modern Cape gooseberry (Physalis peruviana), showing a complete fruit with inflated "lantern" intact and with part of lantern removed to show fleshy fruit within. Right: Fossil groundcherry (Physalis infinemundi, Eocene, Laguna del Hunco flora, Chubut Province, Argentina). Credits: Physalis peruviana (Stefan.Iefnaer, iva Wikimedia Commons, CC BY-SA 4.0); Physalis infinemundi, holotype, MPEF-Pb 6434a, image 61 of 410 (Peter Wilf, via figshare, CC BY 4.0). Images modified from originals.
Explosive dispersal (autochory or bolochory)
Amongst the more novel and exciting ways in which seeds are dispersed is through ballistic or explosive dispersal. In this method of dispersal, the fruit forcibly ejects the seed(s), scattering them for a short distance. The common garden plant Impatiens (also known as balsam, touch-me-not, and jewelweed, amongst other names) is one such plant. It produces capsules. When ripe, an animal brushing by the plant can cause the capsule to open instantly, scattering the seeds. Another plant with dramatic explosive seed dispersal is the squirting cucumber (Ecballium elaterium), which ejects its seeds as the fruit detaches from its stalk.
Ballistic seed dispersal. Video shows ballistic seed dispersal in fruits of Himalayan balsam (Impatiens glandulifera) and squirting cucumber (Ecballium elaterium). Credit: Exploding Cucumbers! (Slo Mo #36, BBC Earth Unplugged, via YouTube).
A spectacular example of explosive dehiscence comes from the tropical sandbox tree (Hura). Upon drying, fruits of this tree explode into pieces, sending the seeds rocketing through the air at up to 70 meters per second (about 230 feet/second) and a distance of up to 43 meters (about 140 feet) from the parent tree (see here)! In addition to the dangers posed by the its exploding fruits, sandbox tree trunks are covered with large prickles and the plant is poisonous; as in some other members of the spurge family (Euphorbiaceae), getting the latex in the eye can cause damage. Perhaps this plant is best avoided.
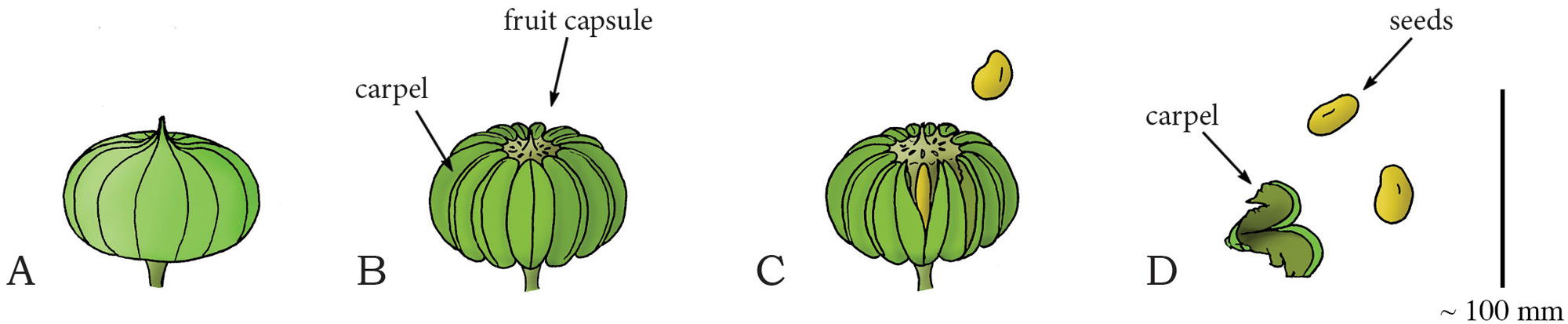
Diagram of the stages in the dehiscence of the sandbox tree fruit (Hura crepitans). A. Immature, dry fruit. B. More mature fruit showing multiple carpels. C. As the fruit dehydrates (loses water), the carpels split open, explosively discharging the seeds. D. An open carpel with seeds. Credits: Image duplicated from and caption modified from Fig. 17 of Sakes et al. (2016) PLoS ONE (CC BY 4.0).
Exploding sandbox tree fruit. Demonstration of explosive sandbox tree (Hura) fruit. It should go without saying: Don't try this at home. Note: This video has no narration. Credit: Hura polyandra explosive seed pod (Mayan Archaeology, via YouTube).
While we obviously cannot observe exploding fruit in the fossil record, we can sometimes infer ballistic seed dispersal in fossil plants based on structural attributes of fossil seeds and fruits as well as plant relationships. For example, many members of the witch hazel family (Hamamelidaceae) have ballistically-dispersed seeds. In these plants, the inner wall at the base of the fruit capsule squeezes the seeds when the fruit is mature, forcing them out of the open fruits. Both fruits and seeds of ballistically dispersed genera in the witch hazel family are found in the fossil record.
Explosive dehiscence of witch-hazel (Hamamelis) fruits. These videos show the explosive ejection of seeds from Chinese witch-hazel fruits (Hamamelis mollis). Whole fruits and fruit endocarps with the outer layers of the fruit wall removed are shown. Note: This video has no narration. Credit: Snippet: This plant can fire seeds with bulletlike force (Science Magazine, from videos by Poppinga et al. 2019 Journal of the Royal Society Interface, via YouTube).
Selected references & further reading
Note: Free full text is made available by the publisher for items marked with a green asterisk.
Academic articles & book chapters
* Atkinson, B.A., R.A. Stockey, and G.W. Rothwell. 2017. The early phylogenetic diversification of Cornales: Permineralized cornalean frutis from the Campanian (Upper Cretaceous) of western North America. International Journal of Plant Sciences 178: 556-566. https://doi.org/10.1086/692766
* Carpenter, J. 2018. Despite living amongst plants with large seeds, extinct giant moa dispersed only tiny seeds. The Conversation. https://theconversation.com/despite-living-amongst-plants-with-large-seeds-extinct-giant-moa-dispersed-only-tiny-seeds-95361
* Carpenter, J.K., J.R. Wood, J.M. Wilmshurst, and D. Kelly. 2018. An avian seed dispersal paradox: New Zealand's extinct megafaunal birds did not disperse large seeds. Proceedings of the Royal Society B, Biological Sciences 285: 2018352. https://doi.org/10.1098/rspb.2018.0352
Collinson, M.E., and P.F. van Bergen. 2004. Evolution of angiosperm fruit and seed dispersal biology and ecophysiology: morphological, anatomical and chemical evidence from fossils. Pgs. 343-377 in: A.R. Hemsley and I. Poole, eds. The Evolution of Plant Physiology, from whole plants to ecosystems. Academic Press. https://doi.org/10.1016/B978-012339552-8/50019-6
Lengyel, S., A.D. Gove, A.M. Latimer, J.D. Majer, and R.R. Dunn. 2010. Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: A global survey. Perspectives in Plant Ecology, Evolution and Systematics 12: 43–55. https://doi.org/10.1016/j.ppees.2009.08.001
* Li, J., C. Song, and C. He. 2019. Chinese lantern in Physalis is an advantageous morphological novelty and improves plant fitness. Scientific Reports 9: article number 596. https://doi.org/10.1038/s41598-018-36436-7
Manchester, S.R., and K.B. Pigg. 2008. The Eocene mystery flower of McAbee, British Columbia. Botany 86: 1034–1038. https://doi.org/10.1139/B08-044
Minami, S., and A. Azuma. 2003. Various flying modes of wind-dispersal seeds. Journal of Theoretical Biology 225: 1–14. https://doi.org/10.1016/S0022-5193(03)00216-9
Poppinga, S., A.-S. Böse, R. Seidel, L. Hesse, J. Leupold, S. Caliaro, and T. Speck. 2019. A seed flying like a bullet: ballistic seed dispersal in Chinese witch-hazel (Hamamelis mollis OLIV., Hamamelidaceae). Journal of the Royal Society Interface 16. https://doi.org/10.1098/rsif.2019.0327
* Palfi, Z., P.G. Spooner, and W. Robinson. 2017. Seed dispersal distances by ants increase in response to anthropogenic disturbances in Australian roadside environments. Frontiers in Ecology and Evolution. https://doi.org/10.3389/fevo.2017.00132
* Sakes, A., M. van der Wiel, P.W.J. Henselmans, J.L. van Leeuwen, D. Dodou, and P. Breedveld. 2016. Shooting mechanisms in nature: a systematic review. PLoS ONE 11(7):e0158277. https://doi.org/10.1371/journal.pone.0158277
* Swaine, M.D., and T. Beer. 1977. Explosive seed dispersal in Hura crepitans L. (Euphorbiaceae). New Phytologist 78: 695-708. https://doi.org/10.1111/j.1469-8137.1977.tb02174.x
* Tiffney, B.H. 1984. Seed size, dispersal syndromes, and the rise of the angiosperms: Evidence and hypothesis. Annals of the Missouri Botanical Garden 71: 551–576. JSTOR (read free with account sign-up): https://doi.org/10.2307/2399037; Biodiversity Heritage Library (free, no account needed): https://www.biodiversitylibrary.org/part/3959#/summary
* Vander Wall, S.B. 2010. How plants manipulate the scatter-hoarding behavior of seed-dispersing animals. Philosophical Transactions of the Royal Society London B, Biological Sciences 365: 989–997. https://doi.org/10.1098/rstb.2009.0205
Wilf, P., M.R. Carvalho, M.A. Gandolfo, and N.R. Cúneo. 2017. Eocene lantern fruits from Gondwanan Patagonia and the early origins of Solanaceae. Science 355: 71-75. https://doi.org/10.1126/science.aag2737
Wilde, V., and M. Hellmund. 2010. First record of gut contents from a middle Eocene equid from the Geiseltal near Halle (Saale), Sachsen-Anhalt, Central Germany. Palaeobiodiversity and Palaeoenvironments 90: 153-162. https://doi.org/10.1007/s12549-010-0028-y
Books & textbooks
Evert R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York.
van der Pijl, L. 1972. Principles of dispersal in higher plants, 2nd ed. Springer-Verlag, New York, Heidelberg, Berlin. https://doi.org/10.1007/978-3-642-96108-3
Websites
* Stuppy, W. (no year). The sadistic dispersal strategy of the puncture vine. Archived blog, Royal Botanic Gardens Kew. https://www.kew.org/blogs/archived-blogs/sadistic-dispersal-strategy-puncture-vine
* Wilf, P. 2017. Image library: Physalis infinemundi Wilf. figshare. https://doi.org/10.6084/m9.figshare.4545787.v1
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen or J.R. Hendricks are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
First released 10 March 2020; last updated 12 June 2020.



