Chapter contents:
Arthropoda
–– 1. Stem-group Arthropods
–– 2. Trilobita
–– 3. Chelicerata ←
–– 4. Mandibulata
The above image shows a fossil eurypterid. Image by Richard Droker; Creative Commons-Attribution-NonCommercial-NoDerivs 2.0 Generic license.
Introduction
The Chelicerata are a diverse group of arthropods—though not as diverse as the Mandibulata—named for the presence of chelicerae. Chelicerae, meaning “claw horn” in Greek, are a pair of appendages anterior to (i.e., in front of) the mouth. In different chelicerates, which include spiders, ticks, mites, scorpions (Scorpiones), horseshoe crabs (Xiphosura), sea scorpions (Eurypterida), and sea spiders (Pycnogonida; following Figure 3 in Giribet and Edgecombe, 2019), the chelicerae take different forms. For example, spider chelicerae are often modified into fangs and are attached to venom glands used to subdue prey, whereas the chelicerae of the Xiphosura (i.e., horseshoe crabs) and the extinct eurypterids (i.e., sea scorpions) often take the form of three-segmented pincers, used in feeding (see image below).
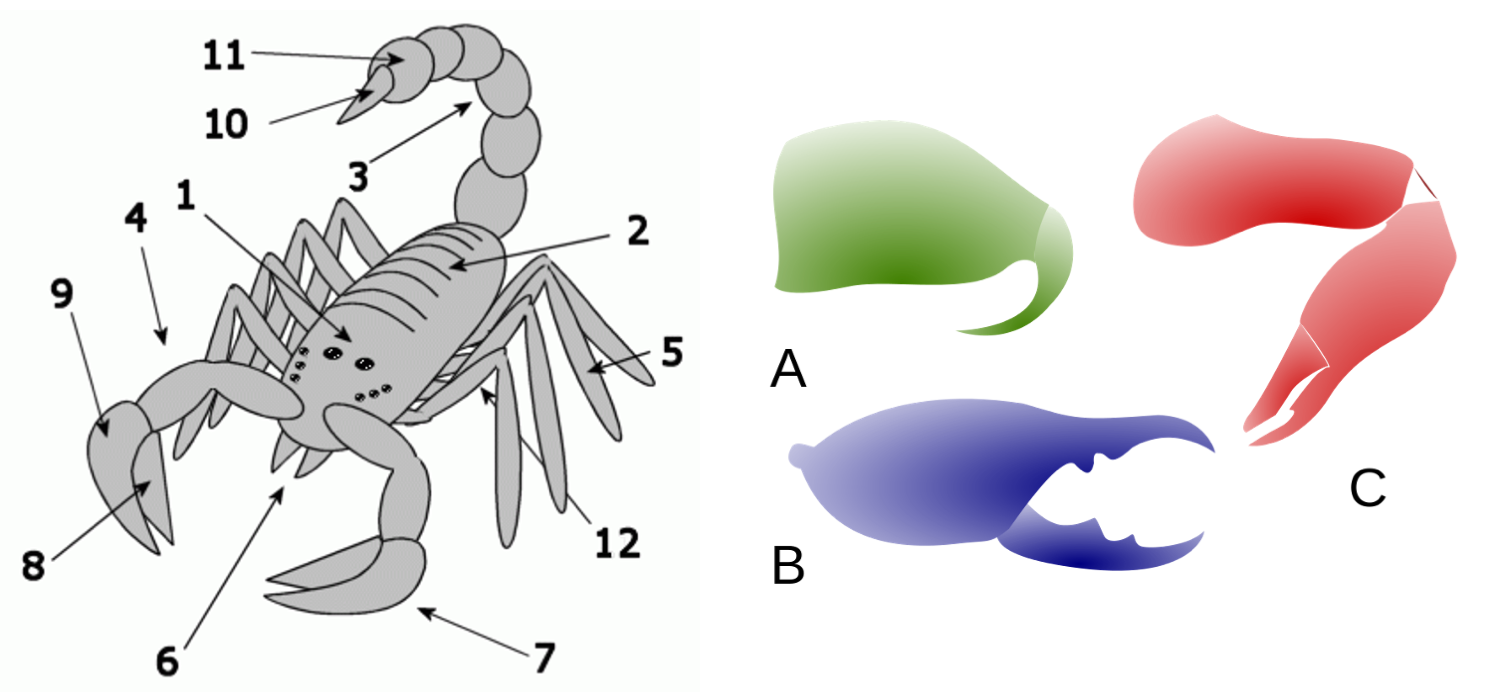
On the left, generalized body parts of a chelicerate, as seem in a scorpion, include: (1) cephalothorax (prosoma); (2) opisthosoma; (4) pedipalps; (5) legs (four pairs); (6) chelicerae; (7) pincers (chelae); (8) moveable claw (manus); (9) fixed claw (tarsus); (10) anus); (12) book lungs (some marine chelicerates used book gills). The mesosoma (tail; 3) is not found in all chelicerates, nor is the stinger (10). On the right are three forms of chelicerae: (A) jack-knife, found in whip scorpions and spiders; (B) scissor, found in camel spiders and false scorpions; (c) and three-segments, found in scorpions, harvestmen, horseshoe crabs, and eurypterids. Left image by Pasixxxx (public domain). Right image by Alexei Kouprianov (public domain).
Within the Chelicerata are two highly diverse groups, Araneae (i.e., spiders, ~45,000 species) and Acari (i.e., ticks and mites; ~50,000 species), and a variety of other less diverse groups (e.g., Eurypterida, Pycnogonida, Scorpiones, and Xiphosura). The general chelicerate body plan includes two major regions, the prosoma and opisthosoma. The prosoma, which is similar to the cephalothorax region in other arthropod groups, is forwardmost and holds the chelicerae, a pair of appendages called pedipalps, and four pairs of walking legs (see image above). Pedipalps, like chelicerae, can take a variety of forms and fill different functions. In spiders, these structures are often used in reproduction; horseshoe crabs use them for locomotion; and, they form the intimidating pincers of scorpions. The opisthosoma, similar to the abdomen of other arthropod groups, typically contains vital organs, such as the heart and respiratory structures. As with the arthropod group in general, there is a high degree of between and within group variation in the body plans exhibited by the chelicerates.
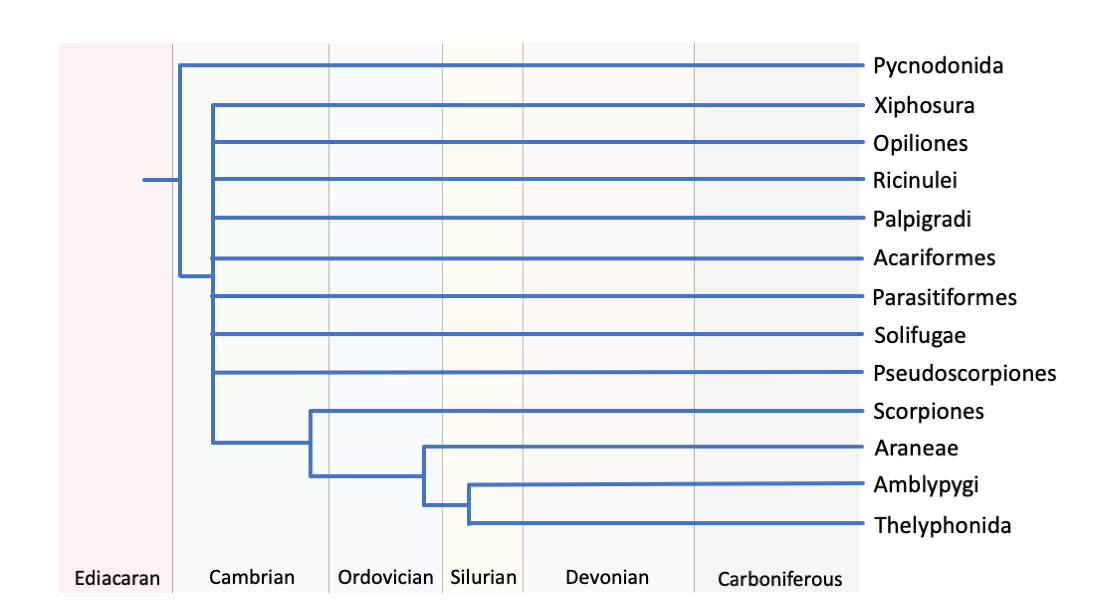
A hypothesis on the phylogenetic relationship of extant clades within the Chelicerata. Note the Acari (ticks and mites), composed of the Acariformes and Parasitiformes, likely is not monophyletic. Redrawn from Figure 3 in Giribet and Edgecombe (2019) in Current Biology.
As described by Giribet and Edgecombe (2019), the relationship between living groups of chelicerates remains uncertain. As seen in the figure above, many chelicerate groups (e.g., Opiliones, Ricinulei, Palpigradi) have undefined branching relationships. Conventionally, pycnogonids (i.e., sea spiders) are considered basal to all other chelicerates (i.e., Euchelicerata), with Xiphosura (i.e., horseshoe crabs) as the next most basal group, splitting from the clade “Arachnida.” This arrangement parsimoniously allows for a single marine to terrestrial transition in the chelicerates. Recent work by Ballesteros and Sharma (2019) has demonstrated, however, that the Xiphosura may in fact be most closely related to the Ricinulei (i.e., hooded tick spiders). This newer grouping presents an interesting scenario, in which the obligatorily marine xiphosurans secondarily evolved their marine lifestyle from a terrestrial ancestry. Though this is not the most parsimonious (i.e., simplest) evolutionary scenario, it does help explain the quasi-terrestrial phase in horseshoe crab reproduction (learn more about horseshoe crabs below). Though there is more confidence in some groupings within the Chelicerata—for example, the tetrapulmonates (i.e., Araneae, Amblypygi, and Thelyphonida) that possess two sets of book lungs—much work remains to resolve the relationships between these clades.
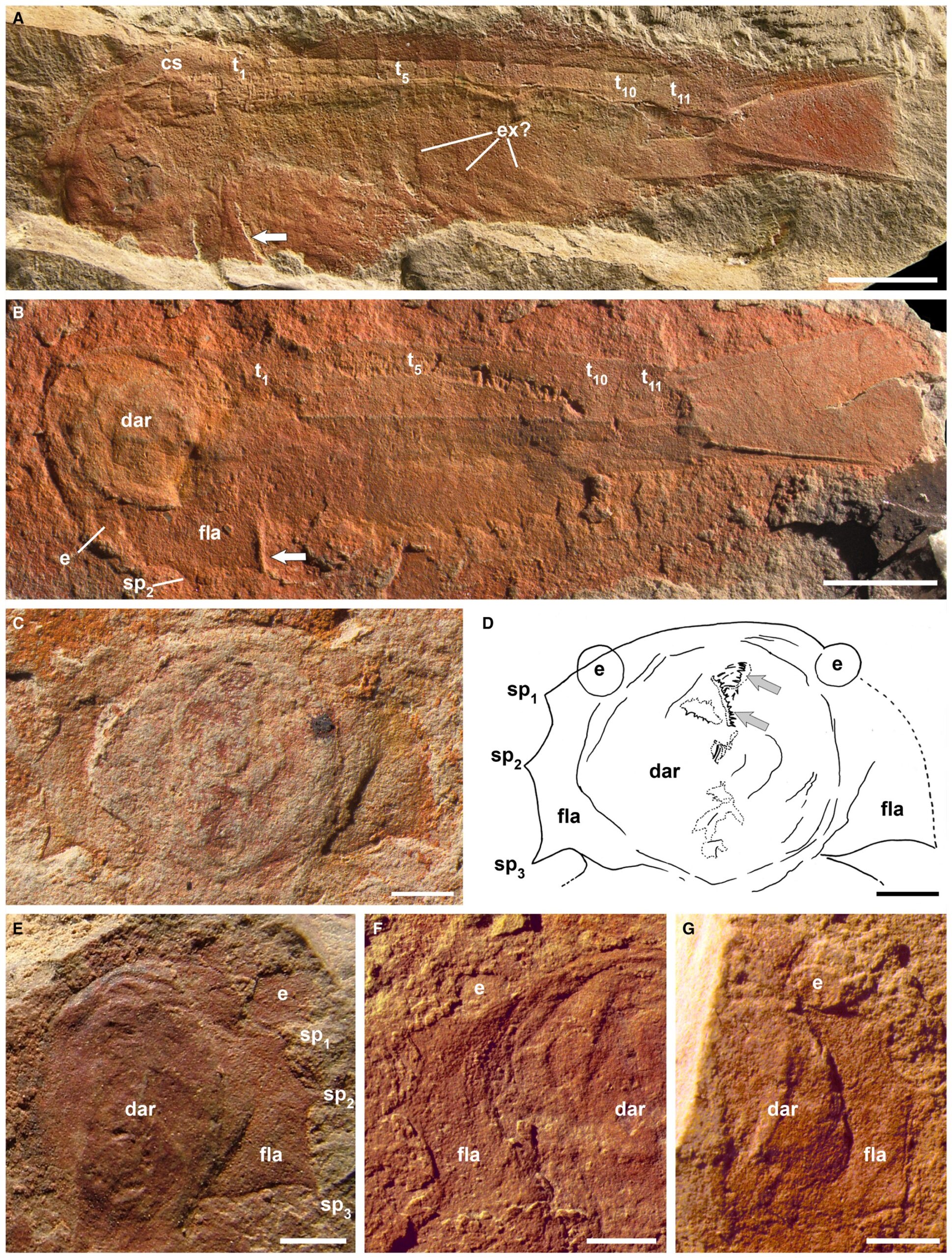
"Wisangocaris barbarahardyae gen. et sp. nov. A, SAM P15228b, almost complete specimen, the arrow indicates the posterior margin of the cephalic shield; possible exopods present on trunk segments 5–9. B, SAM P43679a, holotype, almost complete specimen showing trace of gut and the left eye, the arrow indicates the posterior end of the cephalic shield. C–D, photograph and camera lucida interpretation, SAM P46952a, cephalic shield showing both eyes, the dome shaped axial area, the flat lateral area, three marginal spines and part of a feeding apparatus (arrows), a close up of the feeding apparatus is shown in Figure 4F. E, SAM P46981a, cephalic shield showing the right eye, the dome shaped axial area, the flat lateral area and three marginal spines. F, SAM P49689a, left hand side of cephalic shield showing the left eye, the dome shaped axial area and the flat lateral area. G, SAM P49627a, partial cephalic shield showing the right eye, the dome shaped axial area and the flat lateral area. Abbreviations: cs, cephalic shield; dar, domed axial area; e, eye; ex, exopod; fla, flat lateral area; spn, marginal spines; tn, trunk segments. Scale bars represent 5 mm (A–B) and 2 mm (C–G)." Figure and caption from Jago et al. (2016) in Palaeontology (open access).
In the fossil record, the first known chelicerate is from the early Cambrian, approximately 514 million years ago. Found in the Emu Bay Shale of Australia and described by Jago et al. (2016), Wisangocaris barbarahardyae (see figure above) is similar to other early chelicerates, including Burgess Shale taxa like Sarotrocercus, Sanctacaris, and Sidneyia (view specimens in The Burgess Shale: Fossil Gallery). As discussed by Jago et al. (2016), these taxa are often placed together in a clade based on their morphological similarities and are considered by some to be in the chelicerate crown-group. In the sections below, a variety of other chelicerate fossils—some from extinct groups and others from extant groups—are introduced.
Acari - Mites and Ticks
Acari, which includes what are commonly called ticks and mites, is an extremely diverse subclass of chelicerates with more than 50,000 living species described. Whether this group is monophyletic remains the subject of debate. As shown in the phylogeny below, Acari is often thought to include the orders Acariformes and Parasitiformes. This recent analysis by Lozano-Fernandez et al. (2019) found strong support for this relationship; however, as evidenced by the more conservative phylogeny above from Giribet and Edgecomb (2019), this viewpoint has not yet been commonly accepted. Colloquially, at least, the grouping is supported by similarities in life modes and morphologies. Species in the Acari are often quite small, with even the largest remaining under an inch in length. Like other chelicerates, ticks and mites have a prosoma and an opisthosoma but these two regions are fused together, giving the appearance that these animals lack segmentation in their body plans. Acari are found in most habitats—including terrestrial, aquatic, and marine habitats—and often parasitize other animals, though there are predatory and detritivorous species.
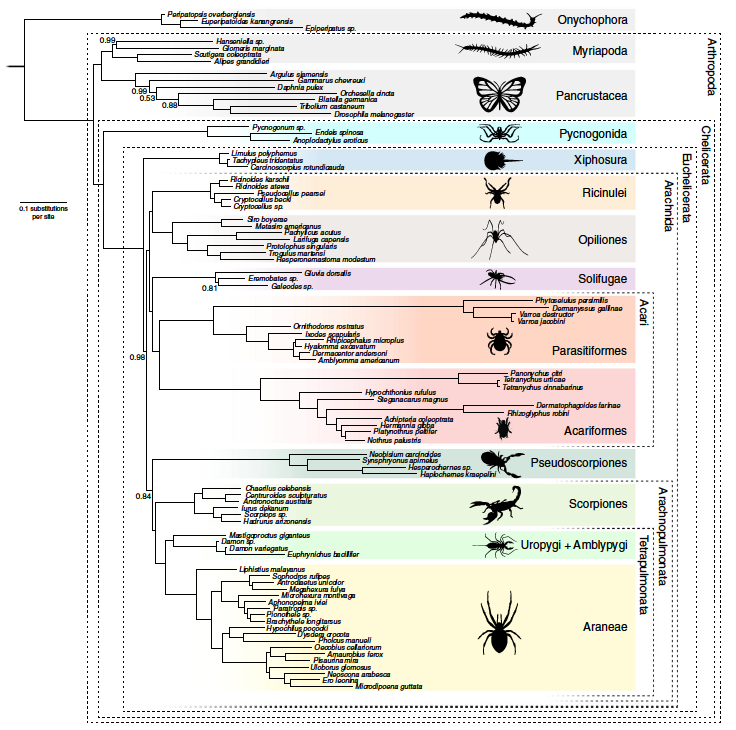
A more explicit hypothesis on the phylogenetic relationships within the Chelicerata. Notably, this hypothesis includes a monophylectic Acari and close relationships between orders (e.g., Ricinulei and Opiliones as sister taxa) left unresolved in the hypothesis presented by Giribet and Edgecomb (2019) above. "Phylogenetic tree derived from the CAT-GTR+G analyses of Matrix A after exclusion of six unstable taxa (Calanus, Bothriurus, Liocheles, Pandinus, Superstitionia and Vietbocap). Removal of unstable taxa did not affect topological relationships but increased support levels at key nodes and improved convergence statistics. Support values represent posterior probabilities. Convergence statistics: Burnin = 3000; Total Cycles =10,000 subsampling frequency = 20. Maxdif = 0.28; Minimal effective size= 63. Silhouettes designed by ART." Figure and caption from Lozano-Fernandez et al. (2019) in Nature Communications; Creative Commons Attribution 4.0 International license.
As reviewed by Sidorchuk (2018), the fossil record of Acari is sparse and is limited to Acariformes during the Paleozoic and most of the Mesozoic. The first Acari in the fossil record is from the Lower Devonian, approximately 410 million years ago. Several other species have been described from the Devonian, including three from an early forest ecosystem of Gilboa, New York (USA). Scattered occurrences have also been reported from the Carboniferous. The record of Acari improves during the Mesozoic, thanks in large part to deposits of amber—fossilized tree resin that often captures small organisms (see an example in the image below). In some cases, the amber fossils are extraordinary, such as the one below showing ticks on a dinosaur feather. During the Mesozoic, extant forms first appeared. More specifically, specimens of the genus Hydrozetes have been reported from 195 million years ago, during the Jurassic. It was not until the Cretaceous, however, that specimens of Parasitiformes first appear, again in amber deposits. With few exceptions, the record of Acari during the Cenozoic is also restricted to amber inclusions.
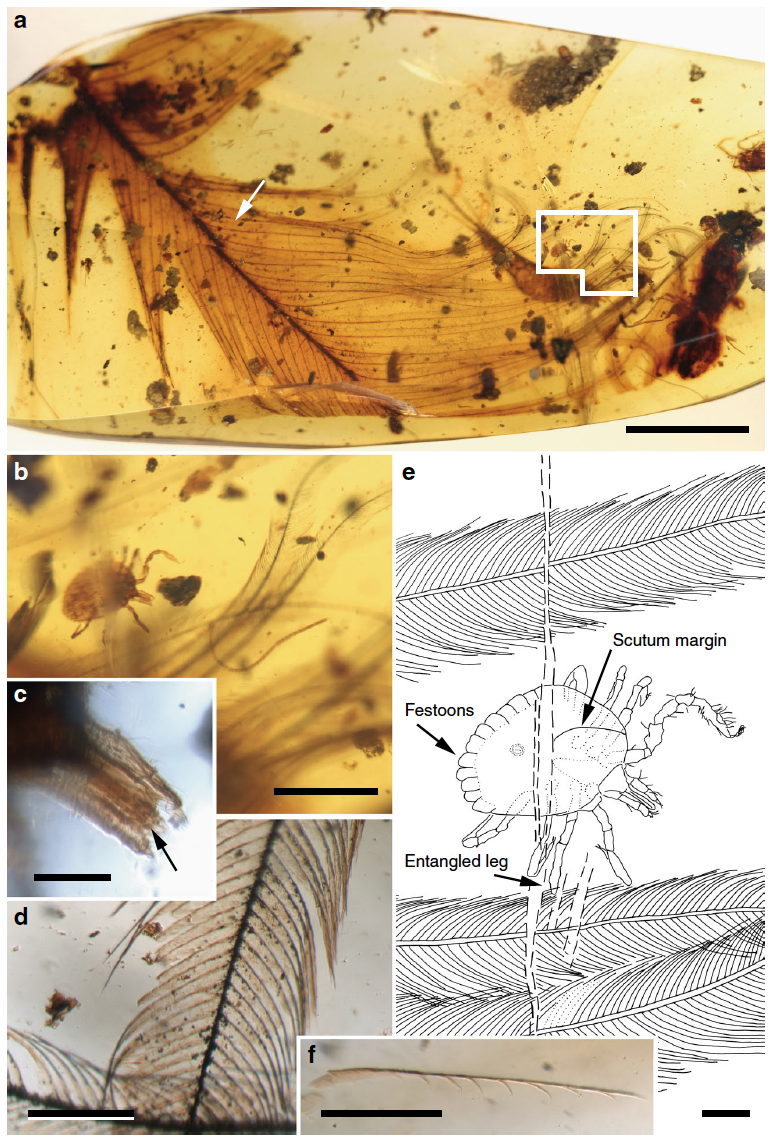
"Cornupalpatum burmanicum hard tick entangled in a feather. a Photograph of the Burmese amber piece (Bu JZC-F18) showing a semicomplete pennaceous feather. Scale bar, 5 mm. b Detail of the nymphal tick in dorsal view and barbs (inset in a). Scale bar, 1 mm. c Detail of the tick’s capitulum (mouthparts), showing palpi and hypostome with teeth (arrow). Scale bar, 0.1 mm. d Detail of a barb. Scale bar, 0.2 mm. e Drawing of the tick in dorsal view indicating the point of entanglement. Scale bar, 0.2 mm. f Detached barbule pennulum showing hooklets on one of its sides (arrow in a indicates its location but in the opposite side of the amber piece). Scale bar, 0.2 mm" Figure and caption from Peñalver et al. (2017) in Nature Communications; Creative Commons Attribution 4.0 International license.
Araneae - Spiders
Spiders are one of the most diverse orders on the planet, with more than 48,000 described living species. Found in almost all terrestrial habitats, and in many aquatic habitats, spiders are numerically the most common predators on land. Spiders exhibit the two-part body plan common to chelicerates, with a clearly distinguished opisthosoma and prosoma. These regions are connected via a structure called a pedicel. As mentioned above, the chelicerae of spiders are modified into fangs, facilitating the predatory lifestyle common in this order. Differentiating them from other arachnids, spiders possess spinnerets, which are used to spin the silk produced by the silk glands.
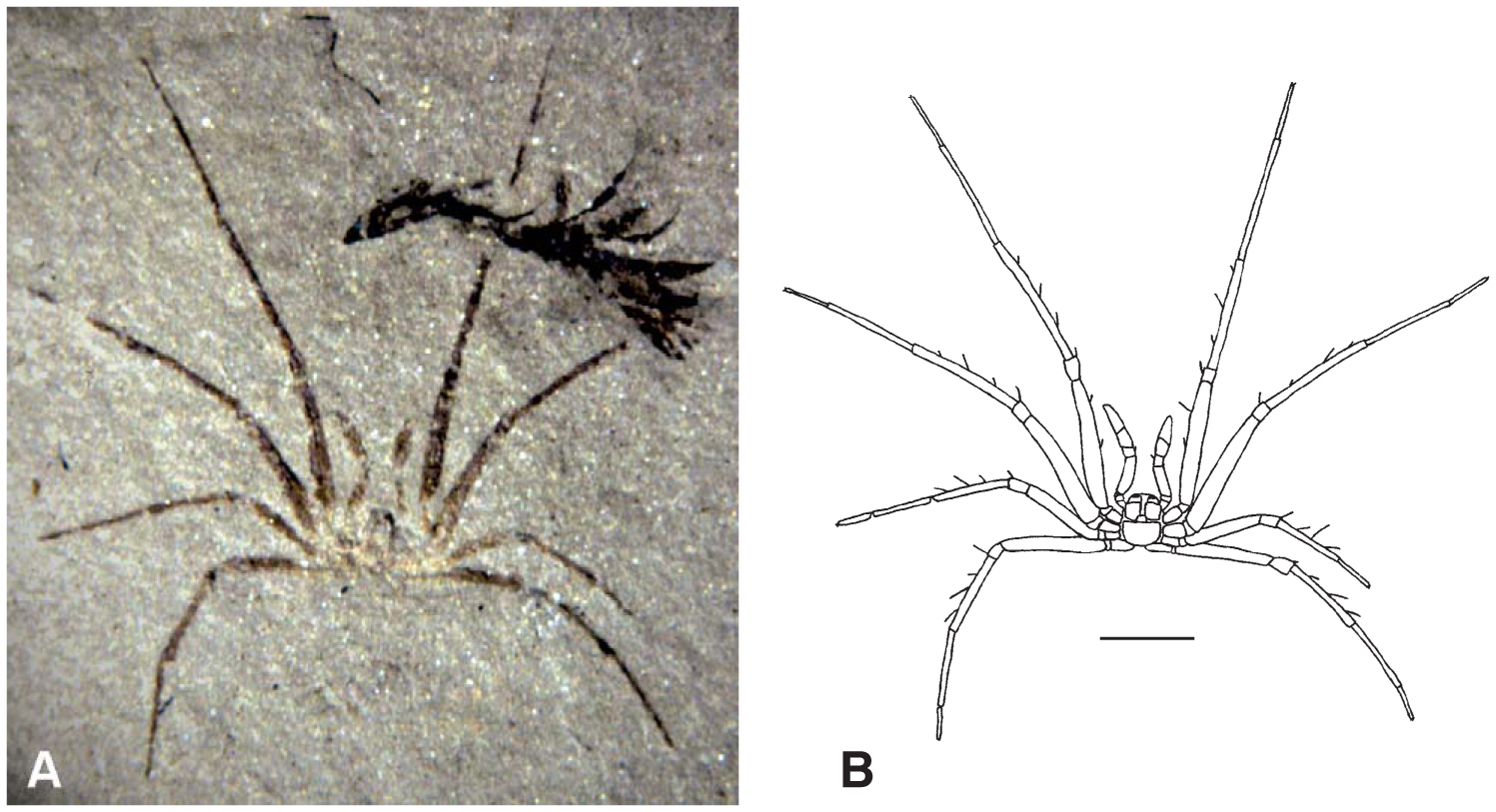
"Holotype of Triassaraneus andersonorum from the Triassic (Carnian) Molteno Formation, Upper Umkomaas ‘Waterfall Locality’ (UMK III), KwaZulu-Natal, South Africa: (A) photograph in incident light under ethanol, (B) camera lucida drawing. Scale bar = 1 mm." Figure and caption from Selden et al. (2009) in African Invertebrates; Creative Commons Attribution 4.0 International license.
The oldest spider known in the fossil record, belonging to the genus Arthrolycosa, is from the Late Carboniferous, approximately 315 million years ago. As discussed by Garwood et al. (2016), this fossil spider is distinguished from other early arachnids by the presence of a spinneret, as other early arachnids (e.g., Idmonarachne brasieri) were very similar but lacked this key feature of the Araneae. By the end of the Paleozoic, one of two extant suborders, the Mesothelae, evolved. Often considered primitive compared to other living spiders, the Mesothelae are represented by only a single family, dwelling exclusively in southeast Asia. The second suborder, Opisthothelae, did not appear until the Triassic. This suborder includes the infraorders Mygalomorphae (e.g., tarantulas) and Araneomorphae (e.g., most familiar spiders like orb, wolf, and crab spiders).
Check out an exceptional amber fossil showing a spider about to eat a meal in this video, "Attacking Spider Caught in Amber 100 Million Years Ago," by Slate (YouTube).
As discussed by Selden and Penney (2017), the fossil record of spiders is relatively sparse, due in large part to the fragile, soft bodies of most spiders. What we know about fossil spiders is derived almost exclusively from Lagerstätten, or deposits with exceptionally well-preserved fossils—the Burgess Shale is a famous example of a Lagerstätten. In the Paleozoic and most of the Mesozoic, these exceptional fossils tend to be encased in sediments. Starting approximately 130 million years ago during the Cretaceous, and from that point onward, most fossil spiders are found in amber, fossilized resin from trees (check out the video above). Many modern spider families are represented in the record from the Eocene onward, suggesting that the Cenozoic spider fauna has remained relatively stable. These families are distinct from those of the Mesozoic and may have evolved earlier than the Eocene; however, amber deposits only become abundant during the Eocene, restricting the potential for study of fossil spiders earlier in the Cenozoic.
Eurypterida - Sea Scorpions
Eurypterids—commonly called “sea scorpions” based on their vaguely similar appearance—were one of the most diverse chelicerate groups during the Paleozoic, with more than 250 described species. The oldest of the eurypterids, recently described by Lamsdell et al. (2015), dates to the Darriwilian (467.3 – 458.4 million years ago) of the Middle Ordovician. Even at this first appearance, the eurypterid, Pentecopterus decorahensis, exhibited derived features, as do other eurypterids from the Middle Ordovician. As discussed by Lamsdell et al. (2015), this evidence suggests that eurypterids either have a longer evolutionary history than is preserved in the fossil record—potentially extending to the Cambrian—or, the clade underwent a rapid diversification after origination during the Ordovician.
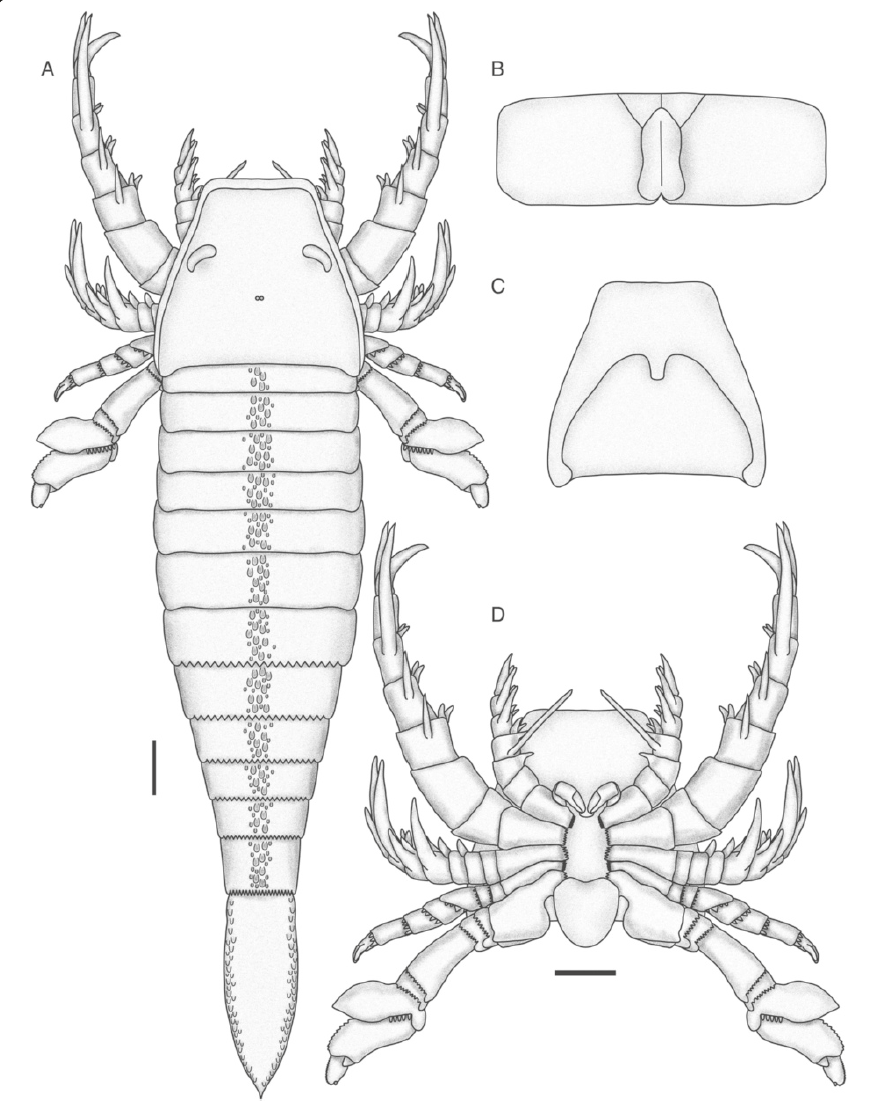
"Pentecopterus decorahensis, reconstruction of adult. a Dorsal view. b Genital operculum. c Ventral view of carapace and prosomal ventral plate. d Ventral view of prosoma. The appendages are shown rotated in lateral view; in life, appendages IV–VI would be oriented so that the anterior edge (in IV) or the posterior edge (in V and VI) of the limb as in the reconstruction would face ventrally. The form of the median and lateral eyes and metastoma are hypothetical and based on ancestral state reconstructions using the phylogenetic matrix and topology. Scale bars = 10 cm (maximum size)." Figure and caption from Lamsdell et al. (2015) in BMC Evolutionary Biology (open access; Creative Commons Attribution 4.0 International license).
Eurypterids reached their peak diversity in the Silurian (see figure below) and, for a time, some species are considered to have been apex predators. These species—belonging to the genera Jaekelopterus and Pterygotus—reached sizes of 2.5 meters and were likely the largest animals in the oceans during the Silurian and beginning of the Devonian. Indeed, Jaelkelopterus, though known from only incomplete specimens, is considered to be the largest arthropod to ever live. Learn more about this giant sea scorpion in the video below.
Learn more about the largest ever arthropods in this video, "Jaekelopterus - The Biggest Arthropod That Ever Lived," by Ben G. Thomas (YouTube).
Large species like Jaekelopterus and stereotypical eurypterids, like Eurypterus (see reconstruction below), belong to the clade Eurypterina, which is one of two major eurypterid clades, along with Stylonurina. The Eurypterina, account for the majority of eurypterid fossils, potentially due to habitat preferences and the broad distribution of some eurypterine species. Indeed, it is the swimming appendage of eurypterines that most readily distinguishes them from the stylonurines, which bear a resemblance to horseshoe crabs in some cases (e.g., Hibbertopterus). In the Eurypterina, the sixth and posterior-most set of appendages form a paddle-like structure used to propel the sea scorpions through the water—compared by some to the swimming limbs of sea turtles. To the contrary, this last set of appendages form limbs for walking in the stylonurines (see reconstructions below).
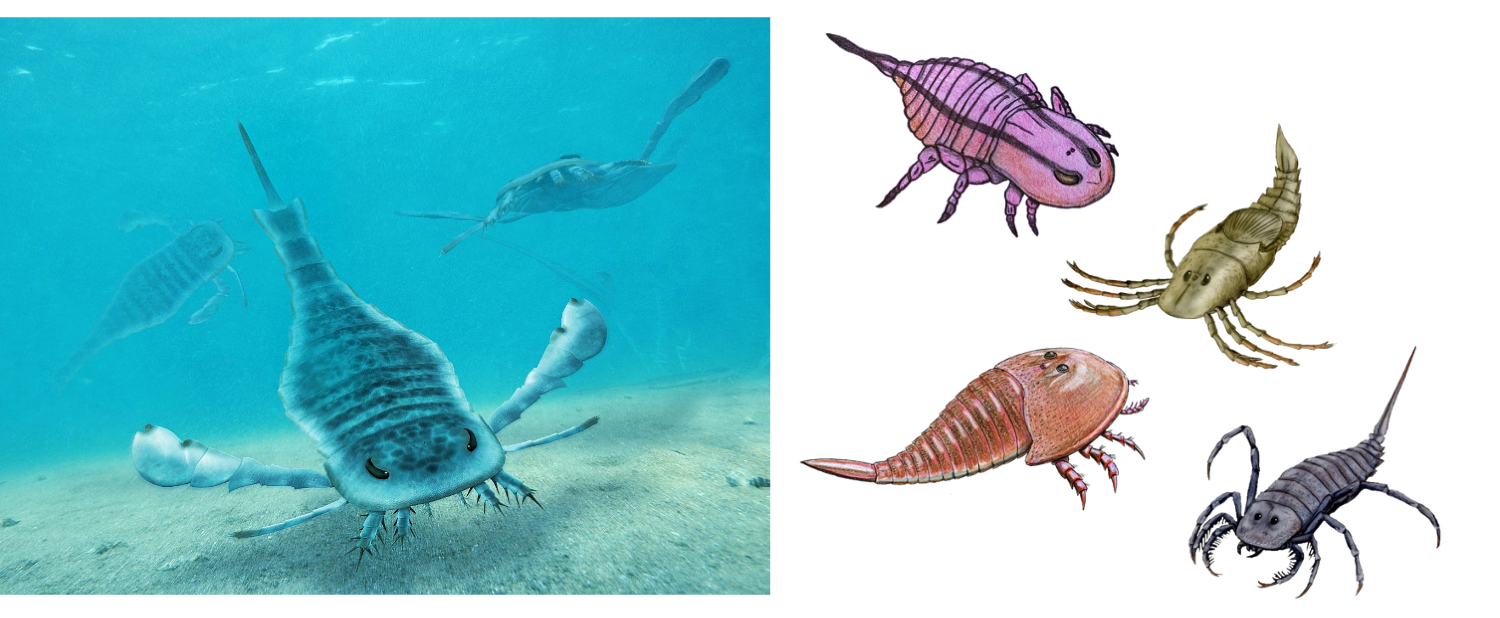
Reconstructions of the Eurypterina, Eurypterus (left), and a variety of Stylonurina (right), including Brachyopterus (top left), Megarachne (top right), Stylonurus (bottom right), and Hibbertopterus (bottom left). Note the large paddle-like appendage of Eurypterus and compare to the hindmost walking limbs in the species at right. Left image by Obsidian Soul; Creative Commons Attribution 2.0 Generic license. Right image by ДиБгд, Ichthyovenator, and Nobu Tamura; Creative Commons Attribution 3.0 Unported license.
Though the earliest known eurypterid (i.e., Pentecopterus) belongs to the Eurypterina, stylonurines from the Middle Ordovician have also been described. Both groups diversified during the Silurian before declining in the Devonian. This decline has been attributed to competition with newly evolving jawless fish during the Devonian; however, this explanation has lost support. For example, analysis of large predatory eurypterines by McCoy et al. (2015) showed a range of ecologies, including species specialized for direct pursuit, scavenging, and ambush predation, and also generalist species capable of multiple strategies. Given this ecological range, McCoy et al. (2015) dispelled the notion that competition with fish led to the demise of the eurypterids. Nonetheless, eurypterids declined during the Devonian, with only one eurypterine clade (Adelophthalmoidea) surviving the Late Devonian Mass Depletion (i.e., extinction). The stylonurines fared somewhat better and even underwent a small diversification in the Carboniferous. Ultimately, all eurypterids went extinct by the end of the Permian.
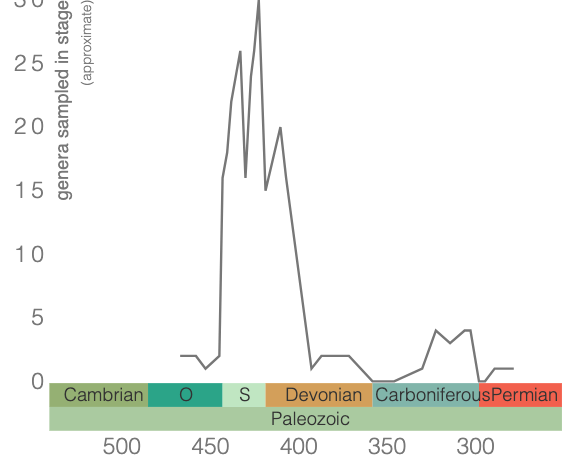
Genus-level diversity of the Eurypterida during the Paleozoic. Figure created using the PBDB Navigator.
In the traditional view of chelicerate phylogenetics, with pycnogonids the first to branch off, eurypterids and xiphosurans (i.e., horseshoe crabs) are considered to be basal to the remaining groups. These remaining groups, referred to as Arachnida—the monophyly of this clade remains dubious—represent a hypothetical, single terrestrial radiation. As mentioned above, new evidence (i.e., a close relationship between Xiphosura and Ricinulei, with a secondary return to marine habitats by xiphosurans) has cast doubt on this traditional view. Even so, the presence of sclerophores (i.e., structures used for sperm-transfer in reproduction) in both eurypterids and arachnids suggests the basal position of eurypterids, with respect to Arachnida, may be warranted. Citing this structure as evidence, along with the potential that some eurypterids were capable of forays onto land, it has been proposed that land-dwelling chelicerates evolved from eurypterids, or at least a common ancestor of eurypterids and arachnids. Other researchers have suggested multiple independent ocean-to-land transitions, leaving the topic open to debate.
Explore the 3D models below to learn more about Eurypterids!
Fossil specimen of the eurypterid Eurypterus remipes from the Silurian of Herkimer County, New York. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Model by Emily Hauf.
Fossil eurypterid (sea scorpion) Dolichopterus macrocheirus from the Silurian of Herkimer County, New York (PRI 42894). Specimen is on display at the Museum of the Earth, Ithaca, New York. Length of preserved portion of specimen is approximately 18.5 cm in length. Model by Emily Hauf.
Fossil eurypterid (sea scorpion) Pterygotus sp. claw from the Silurian Bertie Formation of Herkimer County, New York (PRI 50322). Specimen is on display at the Museum of the Earth, Ithaca, New York. Length of claw is approximately 9.5 cm. Model by Emily Hauf.
Pycnogonida - Sea Spiders
Pycnogonids, also called sea spiders, are an enigmatic group of marine chelicerates. Despite the suggestion of the name “sea spider,” these chelicerates are only distantly related to the terrestrial spiders. The name is instead derived from the spider-like appearance (e.g., long legs attached to a small body; see the video below) of pycnogonids, which did lead early researchers to suggest erroneously a close relationship with terrestrial spiders. As shown in the phylogeny of chelicerates above, the pycnogonids are now commonly thought to be basal to all other chelicerates.
Learn more about sea spider biology and reproduction in this video on "Surprising Facts About Sea Spiders" by Nameless Network (YouTube)
Though more than 1,300 living sea spider species have been described—all belonging to the order Pantopoda—they are represented in the fossil record by a mere thirteen species. Notable fossils include, (1) the oldest pycnogonids, represented by small larvae, from the Upper Cambrian; (2) the oldest adult sea spider, from the Ordovician, likely belonging to the pycnogonid stem group; and, (3) a crown-group sea spider from the Silurian. Additional species are known from the Devonian (see the image below for an example) and Jurassic. Interestingly, the Jurassic pycnogonids—represented by three species—may belong to the order Pantopoda. As discussed by Charbonnier et al. (2007), who published on these Jurassic species, it appears the sea spider body plan and their ecology may have gone relatively unchanged for the last 160 million years.

A fossil sea spider specimen, Palaeoisopus problematicus, from the Devonian. Image by Gheoghedo; Creative Commons Attribution-Share Alike 3.0 Unported license.
Charbonnier et al. (2007) proposed two distinct pycnogonid faunas. One from the Paleozoic and the second from the post-Paleozoic, inclusive of extant species. This scheme, which is seen in other groups of invertebrates (e.g., crinoids; bryozoans), is consistent with morphological differences between the few fossil sea spiders that have been described. The species from the Jurassic have long legs, often associated with species living atop muddy substrate on the ocean bottom. These types of habitats commonly occur deep in the ocean, which is consistent with the depositional environment within which the Jurassic species were found. Although a few fossils pycnogonids have been found in shallower environments, specimens from the Silurian and Devonian are also thought to have lived in the deep ocean. Today, a great many species of sea spider still dwell in the abyssal depths. Sea spiders are also found in shallower habitats (e.g., coral reefs), and it has been proposed that these species may have evolved from deep ocean inhabitants. Watch the video below to see live sea spiders in action!
"Sea Spiders - Deepsea Oddities" by DeepseaOddities (YouTube).
Xiphosura - Horseshoe Crabs
Xiphosurans are better known as horseshoe crabs, though this common name is a misnomer. The xiphosurans are more closely related to spiders and scorpions (i.e., chelicerates) that they are to crabs (i.e., mandibulates). Today, this group is represented by four living species, three are found in Asia and the fourth along the Atlantic coast of the United States. Because of their primitive appearance, which has been largely unchanged since their first occurrence in the Ordovician approximately 480 million years ago, horseshoe crabs are often referred to as “living fossils” (learn more about the biology of this group on the page about living fossils).
Modern specimen of the horseshoe crab Limulus polyphemus from the Atlantic Ocean. Specimen is from the teaching collections of the Paleontological Research Institution, Ithaca, New York. Approximate length of specimen is 28.5 cm. Model by Emily Hauf.
Like other chelicerates, xiphosurans have two main body regions, the prosoma and opisthosoma (see 3D image above). The prosoma forms a rigid shell, the curvature of which resembling a horseshoe, on which the common is based. On the underside, the prosoma also holds the chelicerae and five pairs of walking legs. Moving backwards, the opisthosoma contains vital organs, including the book lungs used in gas exchange. The last part of a horseshoe crab is its telson, which though it appears foreboding, individuals use this structure to right themselves when they get flipped over (watch the video below, at 1m45s, to see this in action).
"Horseshoe Crabs | JONATHAN BIRD'S BLUE WORLD" by BlueWorldTV (YouTube).
Xiphosurans have long been considered as “living fossils,” exhibiting bradytely (i.e., extremely slow rates of evolution), with very little diversity and differentiation between morphologies and ecologies of different species (explore the Jurassic fossil in the 3D model below to understand why). In establishing a phylogenetic framework for this group for the first time, Lamsdell (2016) demonstrated that this traditional view is an oversimplification. Indeed, many of the early analyses responsible for generating this simplistic viewpoint excluded many of the more diverse xiphosuran forms, considering them aberrant specialists. As demonstrated by Lamsdell, it is these aberrant forms that hold the diversity. Most notably, many of these forms occurred after marine xiphosurans transitioned to freshwater habitats during the Paleozoic and Mesozoic—a transition occurring at least five times in this group. As with other groups transitioning to new habitats, the marine to freshwater transition was accompanied by morphological and ecological diversification in the xiphosurans. Even so, the species evolving in these new habitats were likely to be highly specialized and at an elevated risk of extinction. As is borne out in the fossil record, all freshwater forms died out prior to the Cenozoic. Today, only four species of marine xiphosurans, all belonging to Xiphosurida, persist.
Fossil specimen of the horseshoe crab Mesolimulus walchi from the Jurassic Solnhofen Limestone of Germany (PRI 50591). Specimen is on display at the Museum of the Earth, Ithaca New York. Length of specimen is approximately 14 cm. Model by Emily Hauf.
Our understanding of horseshoe crabs and their evolution has also been hindered by a poor understanding of the relationships between taxa included in Xiphosura. On the basis of their similar appearances, a group of organisms called synziphosurines have traditionally been included within Xiphosura (see image below). This grouping is based solely on morphological similarities and, as discussed by Lamsdell (2013, 2016), reflects a somewhat archaic approach to paleontological systematics. Upon reanalysis, Lamsdell has demonstrated that the traditional Xiphosura should be considered a grade—a group of organisms, often paraphyletic, grouped together based on morphological similarities rather than ancestor-descendent relationships—rather than a clade—a monophyletic group of related organisms sharing a common ancestor.

A specimen of Wienbergina, one of the synziphosurine taxa traditionally included in Xiphosura based on appearance. Image by Ghedoghedo; Creative Commons Attribution-Share Alike 3.0 Unported license.
Though several of the synziphosurines do remain at the stem of the xiphosurans in Lamsdell’s phylogenetic hypothesis, many of them are better placed at the base of other chelicerate clades, or unattached to another group. This new definition of Xiphosura includes the Xiphosurida (i.e., the living horseshoe crabs) and their closest extinct relatives. Removal of several synziphosurines from the Xiphosura also has the potential to challenge the traditional view that Xiphosura is basal to the Arachnida. If, in fact, a non-Xiphosuran synziphosurine was ancestral to the arachnids, the placement of Xiphosura in the chelicerate phylogeny may need to be reconsidered. As mentioned above, more recent work by Ballesteros and Sharma (2019) support just such a reorganization. According to their work, Xiphosura may be a sister-group the Ricinulei (i.e., hooded tick spiders), within the Arachnida. Much work remains to be done on chelicerate phylogenetics.
References and Further Reading
Ballesteros, J. A., and P. P. Sharma. 2019. A critical appraisal of the placement of Xiphosura (Chelicerata) with account of known sources of phylogenetic error. Systematic Biology, 68: 896-917.
Usage
Unless otherwise indicated, the written and visual content on this page is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 International License. This page was written by Jansen A. Smith. See captions of individual images for attributions. See original source material for licenses associated with video and/or 3D model content.




