Chapter by:
Jansen A. Smith, Paleontological Research Institution, Ithaca, New York
This chapter was first publicly shared on May 15, 2020.
Chapter citation:
J.A. Smith. 2020. Bryozoa. In: The Digital Encyclopedia of Ancient Life. https://www.digitalatlasofancientlife.org/learn/bryozoa
Chapter contents:
Bryozoa
– 1. Overview
–– 1.1 Phylogenetics
–– 1.2 Anatomy
–– 1.3 Reproduction and Development
–– 1.4 Ecology
– 2. Class Stenolaemata
– 3. Class Gymnolaemata
– 4. Class Phylactolaemata
You can find 3D models of Bryozoa here.
Above image by Christian Schwarz; Creative Commons Attribution-NonCommercial 4.0 International license.
Phylum Bryozoa Snapshot
- Classes: Gymnolaemata, Phylactolaemata, Stenolaemata
- Diversity: ~6,171 extant species, ~17,867 extinct species
- Ecology: marine and freshwater, filter feeders
- Key features of group: largely colonial, lophophore feeding aparatus, cryptic
- Fossil Record: Ordovician to Recent
Overview
Bryozoans are filter feeding invertebrates and can be found in both freshwater and marine habitats, where they are often easy to miss because of their small size and cryptic lifestyle (e.g., encrusting seashells, rocks, or kelp). In almost all species, tiny (< 1-millimeter diameter) bryozoan individuals, called zooids, live together as a colony that often encrusts surfaces, grows branching structures, or, in freshwater species, forms a gelatinous blob. Despite their small size, marine bryozoans are abundant in many fossil assemblages thanks to the preservability of their hard calcium carbonate skeletons. To date, more than 17,800 species of fossil bryozoans have been described and more than 6,000 living species are known. The two primarily marine classes, Stenolaemata and Gymnolaemata, compose the majority of the fossil species, as species in the freshwater class Phylactolaemata do not produce a similarly robust skeleton that would be likely to fossilize.

A variety of fossil bryozoans from the Ordovician of Estonia. Image by Mark A. Wilson (public domain).
Fossils from this phylum of “moss animals”—the translation of bryozoan from Greek—have been found as far back as the Early Ordovician (~480 million years ago). Several studies have reported bryozoans from the Cambrian; however, those findings do not have the support of the entire scientific community. Thus, the time of origin of bryozoans remains contentious and we will use the conservative, Ordovician origination in this chapter. The first definitively bryozoan fossils belonged to the class Stenolaemata and exhibited a high level of morphological diversity. This diversity suggests an evolutionary history predating the Ordovician. Given the absence of skeletal Cambrian fossils—and despite unconfirmed identifications of Cambrian taxa as bryozoans—the earliest bryozoans did not likely have a calcium carbonate skeleton. Consequently, many reconstructions of relationships between bryozoans, such as the one shown below, consider the skeleton-lacking Phylactolaemata to be the most basal bryozoan class (see the phylogeny in the next section).
Fossil specimen of the stenolaemate bryozoan (Order Trepostomata) Spatiopora corticans encrusting the shell of a straight-shelled cephalopod; specimen is from the Ordovician Waynesville Formation of Butler County, Ohio. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Specimen is approximately 8 cm in length. Model by Emily Hauf.
During the Paleozoic, bryozoans, especially stenolaemates, were a common element in marine communities and contributed heavily to the fossil record. Like other shallow marine taxa, the bryozoans were hard-hit by the end-Permian extinction. After a long period of recovery, bryozoans again diversified during the Jurassic and Cretaceous. In the Jurassic and Early Cretaceous, cyclostome stenolaemates dominated the bryozoan fauna but gave way to the newly evolved cheilostome gymnolaemates by the end of the Cretaceous. Likely as a consequence of their adaptable, polymorphic zooids, cheilostomes remain the most abundant bryozoans today. Read more about each of these of the classes—Stenolaemate, Gymnolaemata, and Phylactolaemata—in the sections below.

Examples of Stenolaemata and Gymnolaemata bryozoans. On the left is a fossil Fenestella from the Devonian, belonging to the common Paleozoic stenolaemate order Fenestrida. On the right is a modern Conopeum, encrusting a snail shell, belonging to the gymnolaemate order Cheilostomatida. Left image by Dwergenpaartje; Creative Commons Attribution-Share Alike 3.0 Unported license. Right image by Andre Klicpera; Creative Commons Attribution-Share Alike 3.0 Unported license.
Phylogenetics
The relationship of the bryozoans to other phyla has been revised on several occasions and continues to be an active area of research as more modern genetic data add new information. When originally described, the phylum was called “Polyzoa,” reflecting the colonial nature of this group. From 1831 onwards, the name bryozoan has been used; however, this name originally included an outwardly similar looking group, the Endoprocta. As it was noted in 1869 that these groups shared only a few, coarse external features, they were split as Endoprocta and Ectoprocta. These names are based on the position of the anus relative to the animals’ crown-like feeding apparatuses. In Ectoprocta, the anus is outside, and below the crown whereas the anus is positioned within the crown in the Endoprocta. Despite the splitting of the “bryozoa” into these two groups, the name bryozoa has continued to be used for the Ectoprocta.

A side-by-side comparison of living Endoprocta (left) and Ectoprocta (i.e., Bryozoa; right). Note the similarity of the extended, translucent feeding structures. Left image by Jeff Goddard; Creative Commons Attribution-NonCommercial 4.0 International license. Right image by Christian Schwarz; Creative Commons Attribution-NonCommercial 4.0 International license.
The bryozoans—which is used in this chapter to refer exclusively to the Ectoprocta—were subsequently grouped with the Phoronida and Brachiopoda, in 1891, on the basis of their similar feeding structure, called a lophophore (see image in section below on Anatomy). Now collectively known as the Lophophorata, the group was originally given the name “Tentaculata.” On the basis of molecular data, Bryozoans and Phoronida are often taken to be most closely related to each other, with Brachiopoda forming the most basal clade within the lophophorates. Notably, this new group excludes the Entoprocta, which had been previously considered a close relative of the bryozoans. More broadly, the Lophophorata are nested within the Lophotrochozoa, which does include the Entoprocta and other phyla like Annelida, Mollusca, and Platyhelminthes. This largest group, which itself is nested within Protostomia, is characterized by the aforementioned lophophore feeding apparatus, plus a trocophore larvae—a free-swimming, planktonic larvae with bands of cilia. It is clear that the bryozoans belong within the Lophotrochozoan group; however, at any finer resolution the relationship between bryozoans and the other phyla remains the subject of debate. For example, the phylogenetic hypothesis put forward by Telford et al. (2015) is largely in agreement on the phyla within the Lophotrochozoa, but does not include the Lophophorata grouping, as in the hypothesis from Taylor and Waeschenback (2015). Instead, the phylogeny of Telford et al. leaves the relationship between the "lophophorata" undefined.
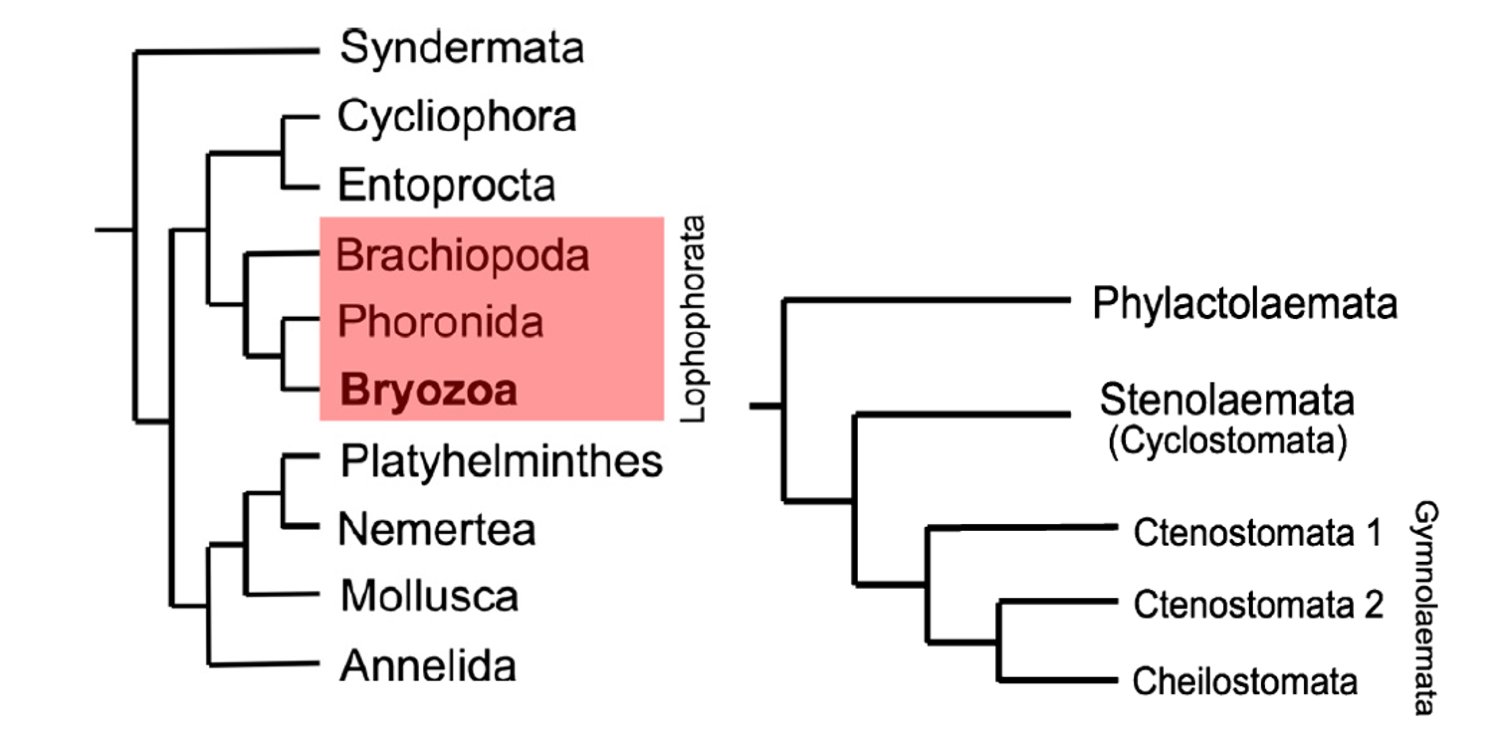
Left image: "Position of phylum Bryozoa within Lophotrochozoa based on the phylogenomic analyses of Nesnidal et al. (2013), showing bryozoans to be the sister group of phoronids, these two phyla in turn constituting the Lophophorata (red box) along with brachiopods." Right image: "Molecular phylogeny of the Bryozoa based on Waeschenbach et al. (2012, fig. 1)." Figures and captions from Taylor and Waeschenback (2015) in Palaeontology under the Permissions of The Palaeontological Association.
Anatomy
The form taken by bryozoan colonies can be highly variable, from gelatinous blobs to upright branching structures and sheet-like encrusters, but the general morphology of a zooid is similar across the classes. Zooids can take several forms (e.g., autozooids, avicularia, heterozooids, kenozooids, and vibracula) but the most common forms in each of the classes are autozooids, which function in feeding the colony and excreting waste. A generic autozooid is shown in the image below, on the right.
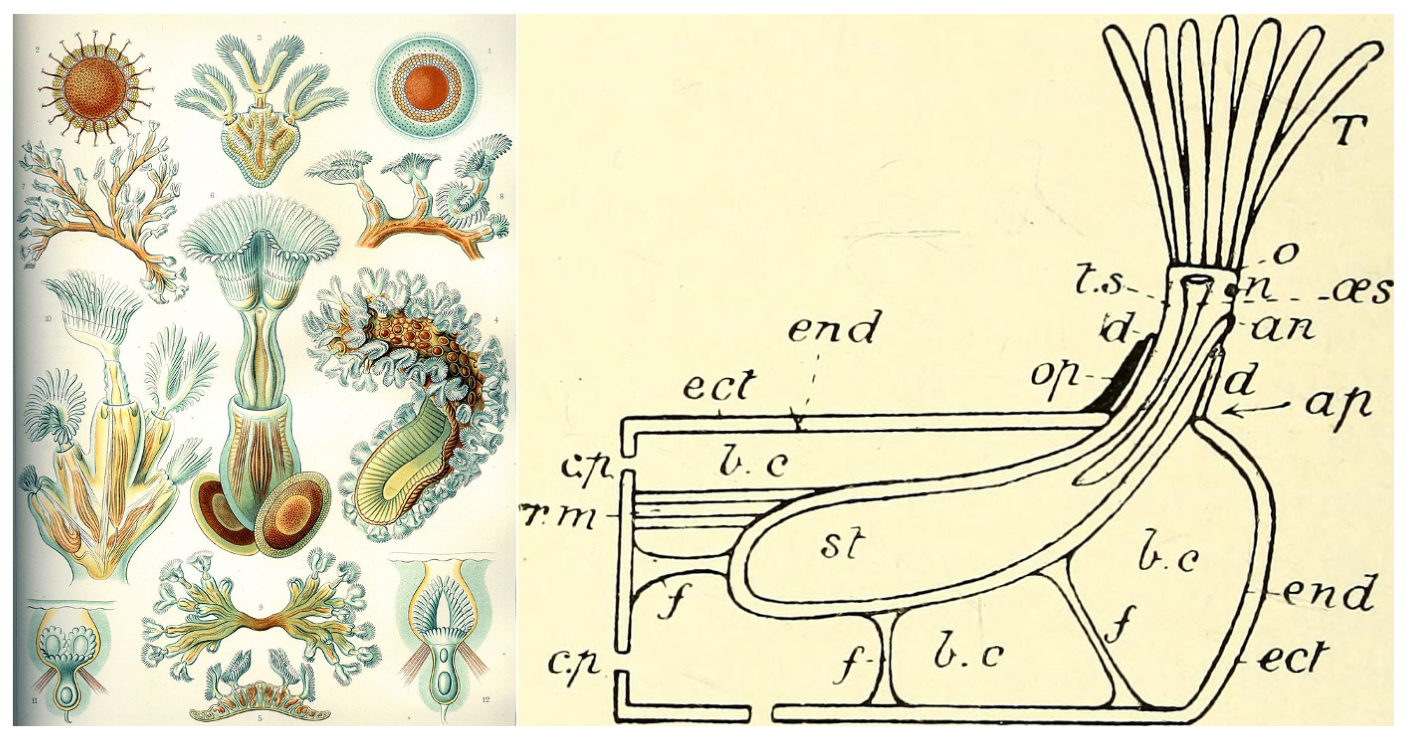
Examples of bryozoans. On the left is a collection of freshwater Phylactolaemata bryozoans, hand drawn by Ernst Haeckel (public domain). On the right is a cross-section view of a generic bryozoan zooid from the British Museum of Natural History's "A Guide to the Fossil Invertebrate Animals" (public domain). The original caption, and key, for the rightside image reads: "Diagram of structure of a typical Bryozoan zooid, as seen in a vertical cut down the middle. an, anus; ap, aperture; b.c, body-cavity; c.p, communication pore; d, diaphragm; ect, ectoderm; end, endoderm; f, funiculi; n, nerve-ganglion; o, orifice [mouth]; oes, oesophagus ; op, operculum; r.m, retractor muscle; st, stomach; T, tentacles; t.s, tentacle sheath." Bolding added for readability.
In autozooids, and most other zooid types, the individual is surrounded in a tissue called a zooecium. The zooecium secretes calcium carbonate to form a skeleton in groups like the cheilostome Gymnolaemata, or gelatinous material in Phylactolaemata (learn more about these groups in the sections below). Sitting in this sheath-like zooecium, autozooids contain a U-shaped gut, with a mouth at the base of the lophophore feeding apparatus and an anus positioned just below the lophophore, hence the name Ectoprocta. In many species, zooids are connected to each other—through an opening labelled as the “communication pore” in the image above—allowing them to share nutrients and transmit chemical signals.
Watch a variety of different bryozoans feed in this video, "Bryozoa - Lophophorata" by Ioly Schwartz. Source: YouTube.
Feeding autozooids, which can be observed in the video above, can extend or retract their lophophores at will. Retraction is controlled by the retractor muscle. Extension of the lophophore is carried out differently in different groups but can be thought of generally as an increase of pressure in the zooecium, forcing the lophophore outward. In some groups, like the cheilostomes, the opening for the lophophore is covered by an operculum, which protects the enclosed individual.

Types of cells found in bryozoan colonies. Autozooids function in feeding and excretion. Avicularium are often defensive structures (see image below). Ovicells are outgrowths from zooids wherein ova (i.e., eggs) are produced and/or gestated. "1-3, Amphiblestrum constrictum Ulrich and Bassler, 1904, holotype USNM 68459, Miocene, Maryland, USA. 1, part of the colony (200 µm). 2, close-up of an autozooid with four distolateral spine bases and an ovicell (100 µm). 3, close-up of the adventitious avicularium (100 µm). 4-6, Amphiblestrum tenuiparietis Canu and Bassler, 1923, holotype USNM 68460, Pliocene, Florida, USA. 4, part of the colony (200 µm). 5, close-up including autozooids, an ovicellate zooid and an adventitious avicularium (200 µm). 6, close-up of a zooid with incomplete ovicell and distolateral spine bases (100 µm)." Original figure and caption from Di Martino et al. (2019) in Palaeontologia Electronica; Creative Commons Attribution Non-Commercial Share Alike 4.0 International license; labels added.
The other types of zooids, which are all technically called heterozooids, do not feed and get their nutrients via their connections to the feeding autozooids. Instead of feeding, heterozooids specialize to perform other functions critical to the survival and reproduction of the colony. Forms like the avicularia and vibracula provide defensive functions. In avicularia, the zooecium and operculum form a beak-like structure that is capable of latching on to potential predators, deterring them from consuming the colony.
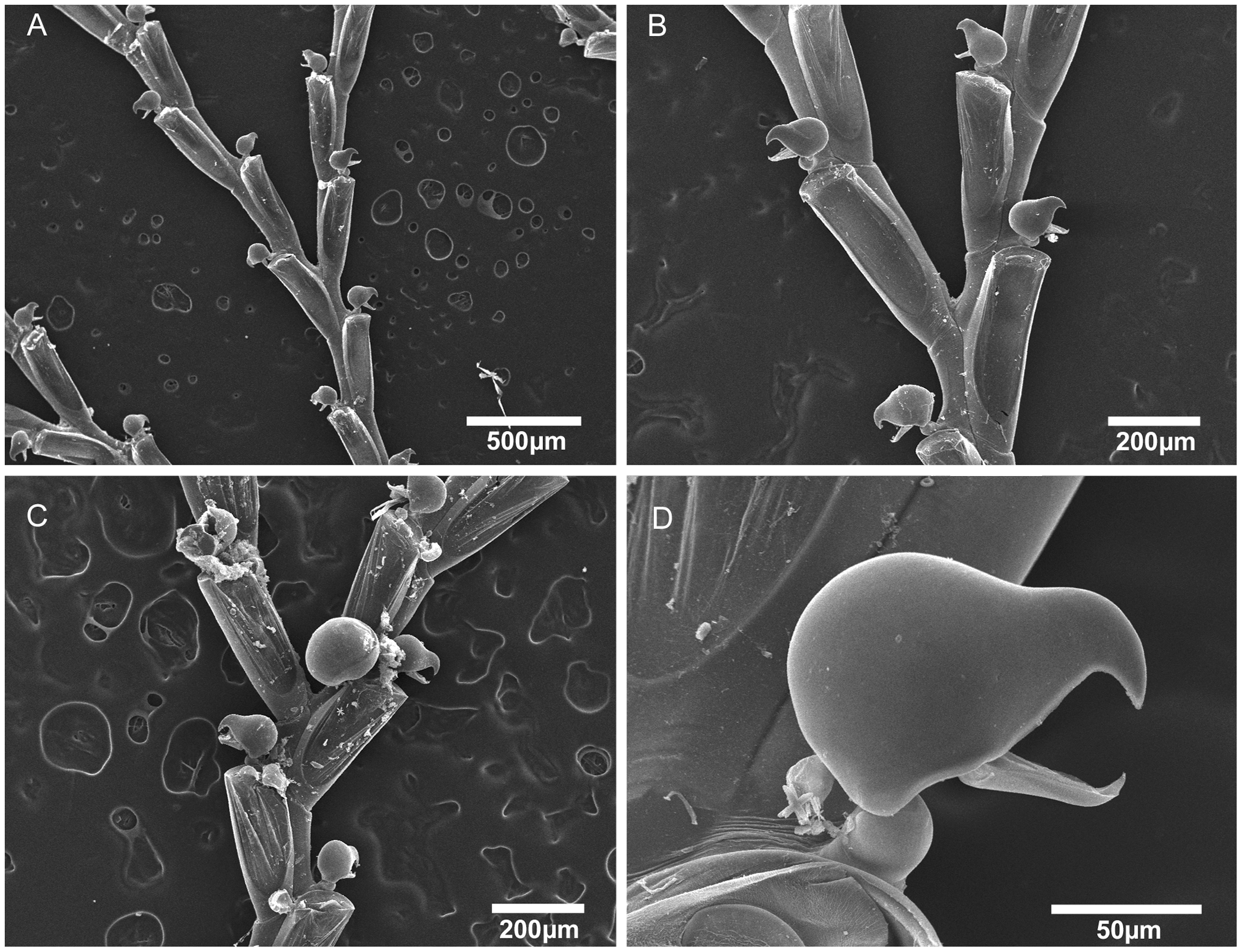
"Scanning electron micrographs of Bugula biota n. sp. Bugula biota n. sp., MZUSP 618, Paratype, Sao Paulo, Brazil, (A) colony, (B) bifurcation, (C) close-up of bifurcation and ovicelled zooids, (D) close-up of avicularium." Figure and caption from Vieira et al. (2012) in PLoS ONE; Creative Commons Attribution 4.0 International license.
Vibracula are long bristles that extend beyond the lophophore (see video below in section on Gymnolaemata). These bristles may play a role in cleaning the colony and also likely provide an early detection system for predators, giving the autozooids time to close before the predator has a chance to consume them (read more about predators of bryozoans below). Kenozooids, the last type of zooid, are employed to strengthen the colony and build structural elements. Depending on the species, kenozooids can also form stalks, holdfasts, spines, and skeletal plates. Though each zooid is an individual, the zooids in a colony often function in tight coordination, almost as if the colony itself was a single individual.
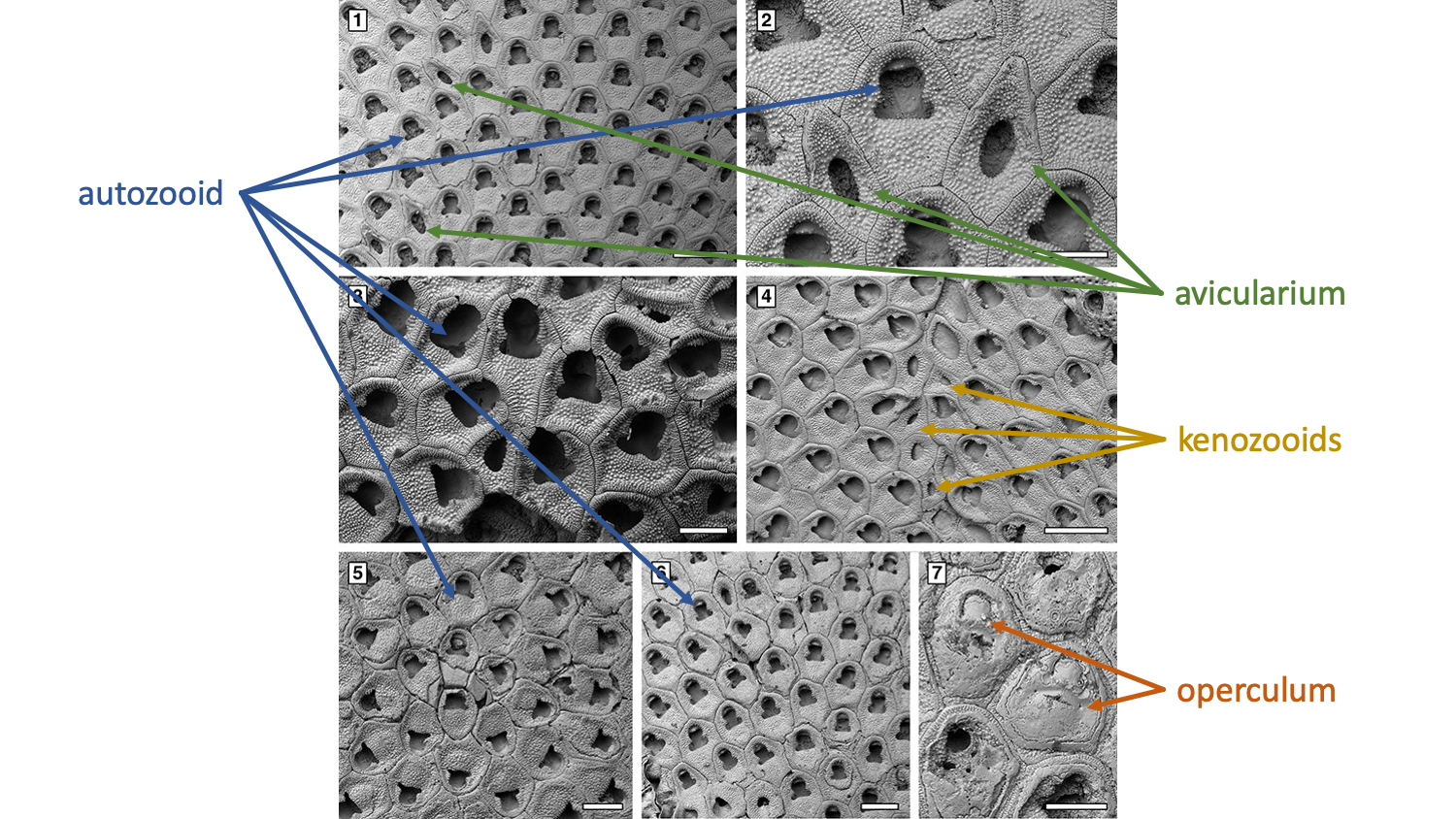
Types of cells found in bryozoan colonies. Autozooids function in feeding and excretion. Avicularium are often defensive structures (see image above). Kenozooids are used for structural purposes, such as creating divisions between colonies. Opercula are used to close individual zooids. "Floridina regularis Canu and Bassler, 1923, Pliocene, lower Tamiami Formation Units 10/11. 1-2, UF 305769 (Shell 1). 1, group of autozooids and vicarious avicularia (400 µm). 2, close-up of an autozooid flanked by two vicarious avicularia (200 µm). 3, UF 305770 (Shell 18), group of fertile zooids (200 µm). 4, UF 305771 (Shell 22), irregularly shaped kenozooids formed at the encounter edge between two abutting colonies (400 µm). 5, UF 305772 (Shell 24), ancestrula and early astogeny (300 µm). 6, UF 305770 (Shell 18), intramural buds (200 µm). 7, UF 305773 (Shell 324), closure plates with scars of the operculum (200 µm)." Original figure and caption from Di Martino et al. (2019) in Palaeontologia Electronica; Creative Commons Attribution Non-Commercial Share Alike 4.0 International license; labels added.
Reproduction and Development
Bryozoans are capable of both sexual and asexual reproduction. Asexual reproduction is the mode by which most colonies grow. New zooids bud from older zooids, expanding the colony outwards (or upwards). You can watch this process in the video below. The freshwater Phylactolaemata also asexually produce statoblasts (read more in the Phylactolaemata section below), which are hardy, chitin covered cells masses capable of surviving harsh conditions and starting new colonies.
Watch as a bryozoan, Bugula neritina, goes through its life cycle. "Bugula neritina - the life cycle of a marine bryozoan" by Alvarro Migotto. Source: YouTube.
When reproducing sexually, zooids produce eggs and sperm. In some groups—and likely in primitive, ancestral Paleozoic bryozoans—both eggs and sperm are released into the water, where they form a free-swimming larva, and eventually new colonies. These colony founders are called ancestrula, and the entire new colony will form, as clones, around this first individual. In many modern bryozoans, eggs are not released into the water column. Instead, sperm are released by sperm donating individuals and are captured by the lophophores of egg donating individuals. In the marine groups, individuals are sequential hermaphrodites, most often transitioning from sperm donors to egg donors during their life. In contrast, freshwater bryozoans are simultaneous hermaphrodites, with all individuals capable of producing both sperm and egg. After the sperm are captured from the water column, fertilized eggs are held within the colony until they transform into free-swimming larvae. This brooding behavior may improve the reproductive success of bryozoans and the evolution of this behavior has been linked to the diversification of post-Paleozoic bryozoans. After settling in a new location, larvae undergo a complete metamorphosis as they turn into an ancestrula. Watch the video above to see this process in action!
Ecology
Bryozoans can be found in most aquatic habitats. In freshwater systems, Phylactolaemata bryozoans are often among the most abundant invertebrates. Though the class is primarily composed of marine species, a few Stenolaemata also occur in freshwater. The third class, Gymnolaemata, is exclusive to marine environments and, by number of species, is the most diverse class of bryozoans. Regardless of environment, all bryozoans feed by filtering tiny particles from the water column using their lophophores. The vast majority of bryozoans are colonial, though several exceptions exist, such as the aptly named genus Monobryozoon.
In their various environments, bryozoans are occasionally consumed by predators, such as the nudibranch in the video below. Predators like nudibranchs, sea urchins, and sea stars move along the surface of a colony and consume individual zooids. Predation of this type can induce the formation of defensive structures (e.g., spines) by the colony. Anti-predatory defenses can be energetically costly to produce so some species (e.g., Membranipora membranacea) only produce them when there is an immediate threat (i.e., predator present). As demonstrated in laboratory experiments by Drew Harvell (1984), spines can take up to two days to form, meaning that individuals zooids will be consumed—before and while defenses are produced—but the colony will most likely survive. Predation of this type may have contributed to the evolution of specialized zooids like the vibricula and avicularia.
A very well camoflagued nudibranch (shell-less snail) consumes individual zooids as it moves across the colony. Predation such as this can induce the growth of defensive spines by the bryozoa. "Nudibranch predator (Corambe steinbergae) and bryozoans" by Spineless Studio. Source: YouTube.
Erect bryozoan forms—as opposed to encrusting—are also under threat from larger predators, like fish, that are liable to consume large portions of a colony, if not the entire colony. As discussed by McKinney et al. (2003) in their review of predation on bryozoans, several fish species have been observed feeding on the species Bugula neritina (featured in the video above on development). Bryozoans may also be subject to incidental predation, as small species of fish, shrimp, and crab can be found living amongst the branches of erect forms.
A variety of organisms, like shrimp, fish, and crabs, can often be found living within erect bryozoans. "Blending In - Lacy Bryozoan and its Inhabitants" by Bubble Vision. Source: YouTube.
Though predation on bryozoan was also likely common in the geological past, evidence of such predation is sparse in the fossil record. This sparseness may be due to the mode of predation and the small size and fragility of bryozoans. For example, many predators crush prey when they consume it, leaving little evidence of the interaction. When feeding occurrs on the surface of bryozoans, as shown in the nudibranch in the video above, there may not be any trace of predation. There are notable exceptions, however, such as predation by sea urchins. The mouth parts of a sea urchin, which form a structure called Aristotle’s lantern, leave rasping scratch marks on the surface of bryozoan colonies. These types of traces have been found from the Triassic onward. There is also some evidence to suggest that shell-boring predators, such as muricid and naticid snails (read more about drilling predation), consume bryozoans. The purported bore holes in bryozoans are quite small, however, and it is challenging to assign them to a particular type of trace making predator. Though the direct evidence of predation may be sparse, trends for thick, robust skeletons and specialized defensive zooids suggest that predation has played an important role in the evolution of bryozoans.
Class Stenolaemata
Stenolaemata are the first bryozoans to appear in the fossil record, dating to the Early Ordovician approximately 480 million years ago. Though they are the first in the record, they were not likely the first of the three classes to evolve. As discussed by Taylor and Waeschenbach (2015) in their review of the topic, the first Stenolaemata may have arisen through the biomineralization (i.e., skeletonization) of the outer wall of an early gymnolaemate. This is only one proposed hypothesis of relationship, however, as other phylogenies have placed Stenolaemata basal to the Gymnolaemata. Regardless of the relationship between these two classes, Phylactolaemata is very likely basal to both (see the phylogeny above). Within the Stenolaemata, a handful of orders were present during the Paleozoic, but only a single order, Cyclostomata, survived beyond the Triassic and to the present.
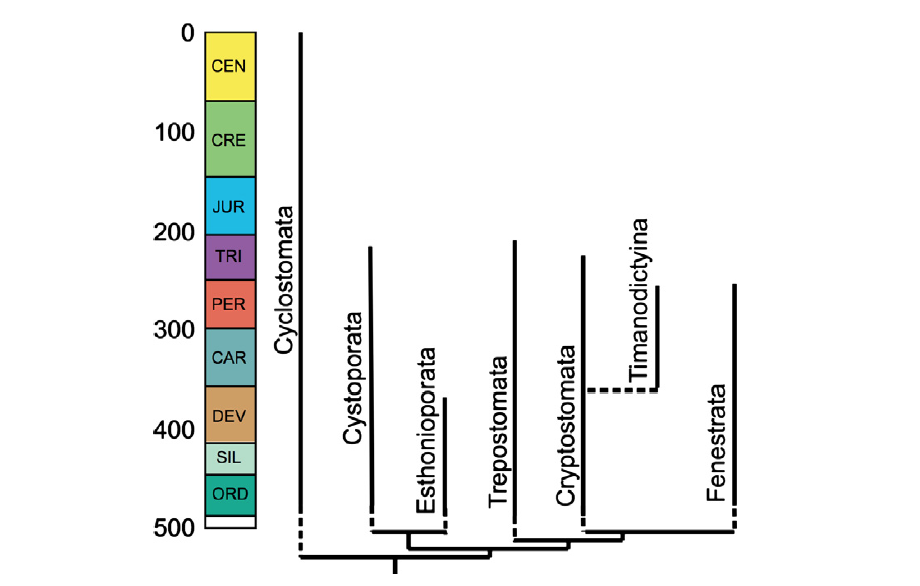
"Stenolaemate bryozoan tree with known ranges of orders. Tree topology as in Figure 5 but with the addition of Timanodictyina, which may nest within Cryptostomata (Jimenez-Sanchez et al. 2010). The fossil record of all orders apart from Timanodictyina begins in the Early or Middle Ordovician, accounting for the shortness of the ghost ranges (vertical dashed lines)." Figure and caption from Taylor and Waeschenback (2015) in Palaeontology under the Permissions of The Palaeontological Association.
During the Paleozoic, the Stenolaemata were the dominant class of bryozoans and an important component of early reef structures and benthic habitats. In addition to branching and encrusting forms (e.g., Polypora), these early bryozoans also formed mound-like structures (e.g., Prasopora) and more eccentric forms such as the corkscrew-like Archimedes. Check out the 3D models below to explore these stenolaemates.
Fossil specimen of the stenolaemate bryozoan (Order Fenestrida) Polypora elliptica from the Pennsylvanian Thrifty Formation of Eastland County, Texas (PRI 76722). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Maximum dimension of specimen is approximately 4.5 cm.
Fossil specimen of the stenolaemate (Order Trepostomata) bryozoan Prasopora sp. from the Ordovician Maquoketa Shale of DeKalb County, Illinois (PRI 76720). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York.
Fossil specimen of the stenolaemate (Order Fenestrida) bryozoan Archimedes wortheni from the Carboniferous (Mississippian) Warsaw Limestone of Hancock County, Illinois (PRI 70774). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 11 cm.
Stenolaemates—and bryozoans, in general—were hard hit by the end-Permian extinction and are rare in Triassic-aged rocks. All orders of Stenolaemata, except Cyclostomata, went extinct by the end of the Triassic. During the Jurassic, the cyclostomes began to radiate and dominated the bryozoan fauna until the end of the Cretaceous. As shown in the figure below, Cyclostomata diversity in the Jurassic peaked with approximately 30 genera, and 80 species, before declining at the end of the period. This end-Jurassic decline may be an artifact of poor preservation, however, as many of the same genera reappear again in the Early Cretaceous after several million years of absence from the record. The end of the Cretaceous saw a peak in cyclostome genetic and morphological diversity, as the group exceeded 170 genera. Though still extant today—composing less than 10% of modern bryozoan species diversity—cyclostomes were supplanted as the dominant component of the global bryozoan fauna by the Cheilostomata gymnolaemates after the Cretaceous-Paleogene mass extinction, a trend that was already beginning prior to the extinction during the latest Cretaceous.
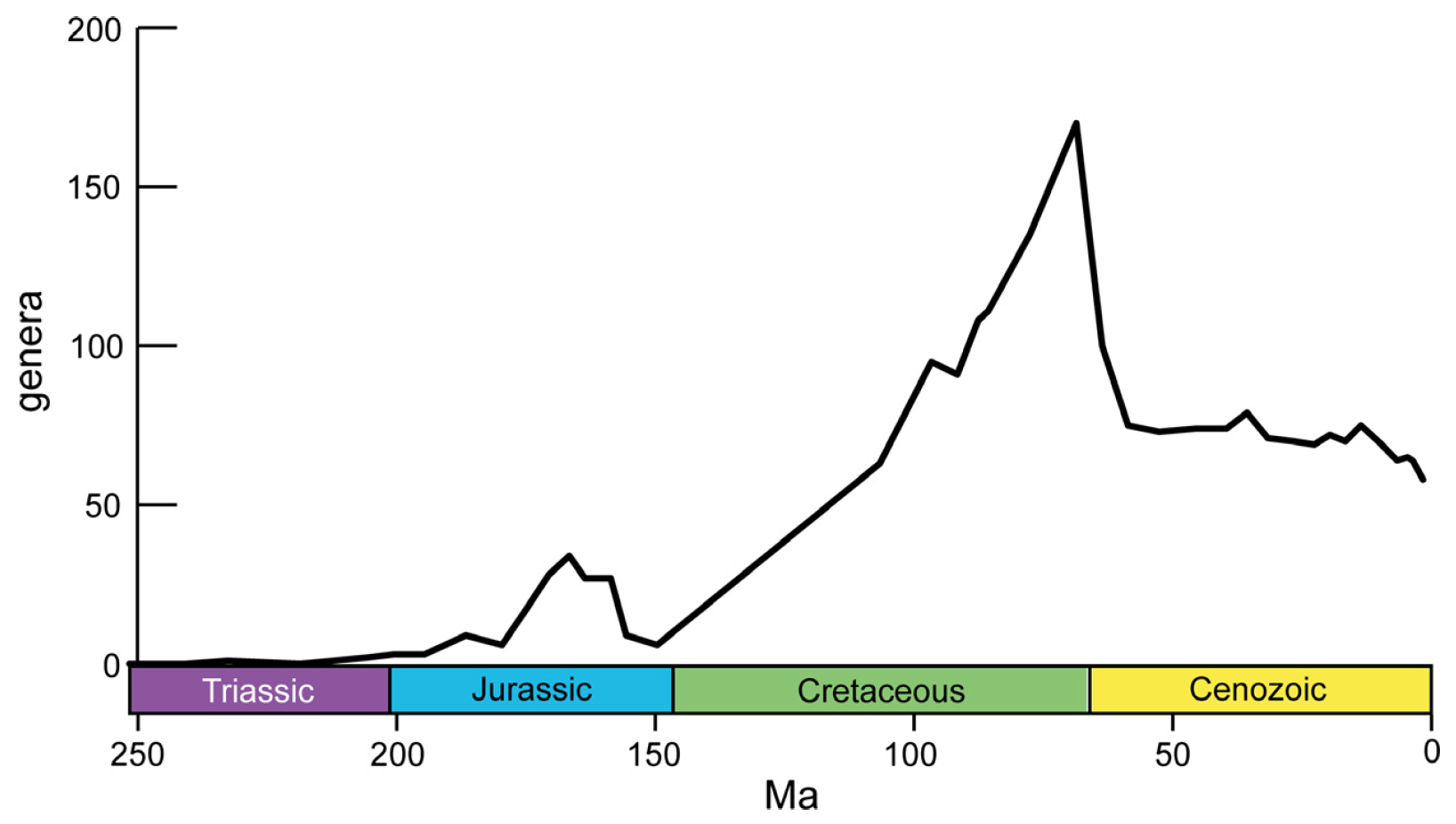
"Generic diversity of post-Palaeozoic cyclostomes based on stage-level data published by Powers and Pachut (2008) for the Triassic, Taylor and Ernst (2004) for the Jurassic, and McKinney and Taylor (2001) for the Albian–Pleistocene (no compilation exists for the Berriasian–Aptian interval). Note the diversity peaks in the Bathonian and the Maastrichtian, followed by a crash at the KT boundary and in the early Paleocene, and a modest overall subsequent decline towards the present day." Figure and caption from Taylor and Waeschenback (2015) in Palaeontology under the Permissions of The Palaeontological Association.
Class Gymnolaemata
Gymnolaemates are the most abundant and diverse bryozoans today, comprising at least 80% of known bryozoan species. This abundance has only come about relatively recently, with the evolution of Cheilostomata in the Jurassic and diversification of this now-dominant order during the second half of the Cretaceous. The second major order of Gymnolaemata, the Ctenostomata, date back to at least the Middle Ordovician; however, species in this order do not form hard skeletons and thus have a sparse fossil record. Though they do not produce skeletons, ctenostomes do leave characteristic boring traces on the substrates they occupy, making it possible to infer their presence in fossil assemblages (see the image below).
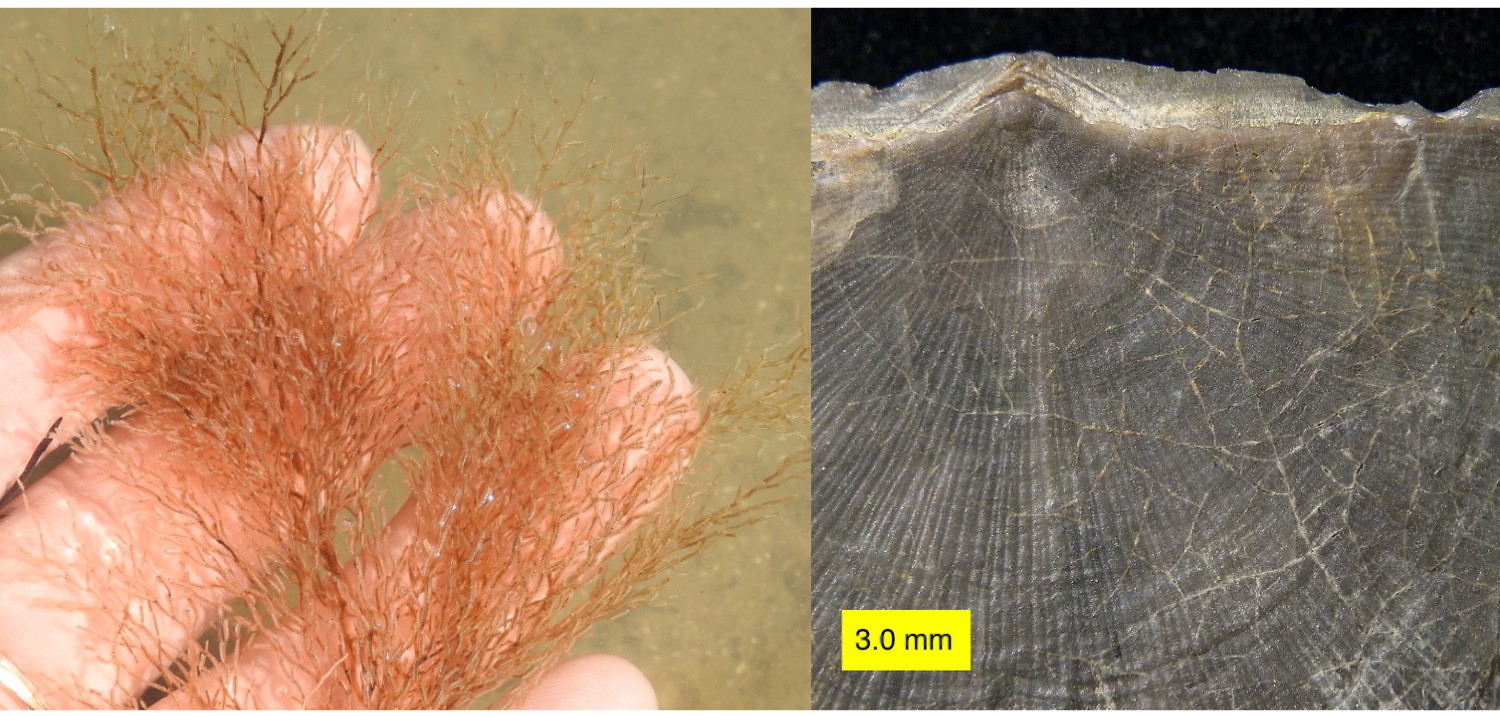
On the left is a living Ctenostomata, Amathia alternata, and on the right is an Ordovician brachiopod shell with traces from Ropalonaria venosa on its surface (the thin tan lines, primarily on the right side of the shell). Left image by Sam Kischnick; Creative Commons Attribution-NonCommercial 4.0 International license. Right image by Wilson44691; Creative Commons CC0 1.0 Universal Public Domain Dedication.
Within the Gymnolaemata, it is likely that Cheilostomata arose from the Ctenostomata during the Jurassic. Because they do not have a skeleton or a good fossil record, it has been more challenging to determine when and how the ctenostomes diverged from the other early bryozoans, though it occurred by at least the Middle Ordovician and potentially earlier in the Cambrian. Complicating matters further, it is very likely that the ctenostomes are not a monophyletic group and some evidence from living species has even suggested that they are more closely related to the Stenolaemata. Likewise, though it is often considered a monophyletic group, the speciose Cheilostomata may be polyphyletic. Like other small, invertebrate organisms, bryozoans are considerably understudied, and much more extensive sampling and analysis of living species will be needed to parse out these relationships.
Like the broader phylogeny, the relationship between different cheilostomes remains unclear. Regardless of their relationships to each other, all species in this order share two common traits. An operculum, the first trait, is used to close individual zoecium when the lophophore has been retracted, serving as a passive defensive mechanism against predators or to limit exposure to unfavorable conditions. In some groups, the operculum has evolved into more active defensive mechanisms, like mandibles to clamp onto predators or a long bristle to aid in sensing threats (and potentially to help keep the colony clean). This innovation likely played an important role in the evolution of this group, and ultimately its success, as predation escalated during the Mesozoic while these forms were evolving. The second trait shared by this group is a biomineralized skeleton, which also would have made individuals more resilient to predation. This is the second time a biomineralized skeleton evolved in bryozoans—the first was by the early Stenolaemata.
Fossil specimen of the cheilostomate bryozoan Cellepora sp., which has encrusted the shell of a gastropod; specimen is from the Neogene Yorktown Fm. of Hampton, Virginia (PRI 76724). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Maximum length of specimen is approximately 2.5 cm. Model by Emily Hauf.
Cheilostomes are traditionally split into two suborders: Anasca and Ascophora. In addition to subtle differences in the mechanism used to retract and extend their lophophores, these two suborders differ by the presence of a calcified frontal shield in the Ascophora (see the scanning electron images below to compare and contrast Mesozoic anascans and ascophorans). Based on their anatomical differences, anascans are considered to be the more primitive group, which is verified by their presence in the fossil record 50 million years before the ascophorans.
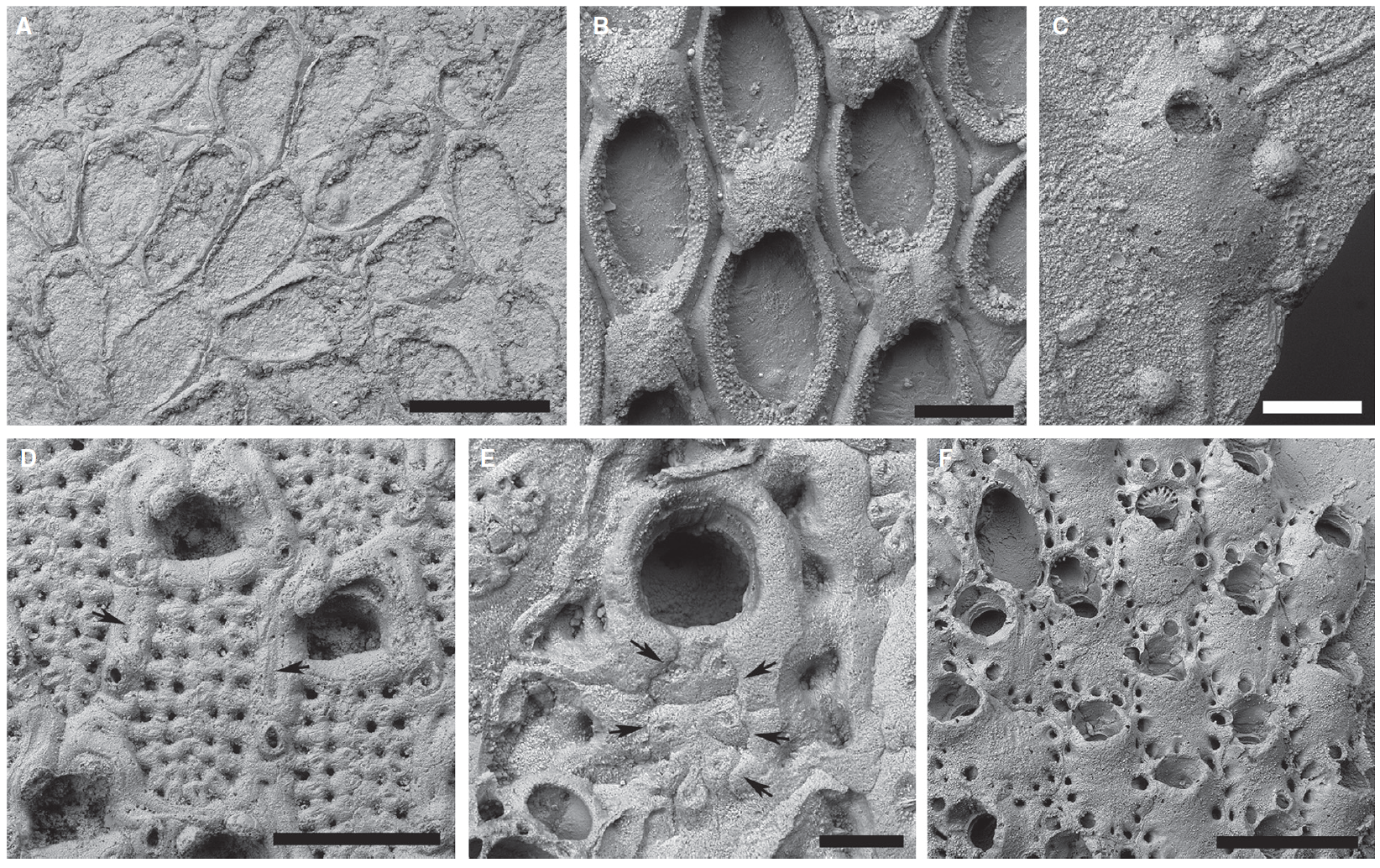
"Scanning electron micrographs showing morphological disparity among Mesozoic cheilostomes. A, Pyriporopsis pohowskyi Taylor, holotype, NHMUK BZ766, Upper Jurassic, Madbi Fm., Jebel Sakha; the oldest known cheilostome, a malacostegine anascan. B, Wilbertopora listokinae Cheetham et al., NHMUK BZ1352, Cenomanian, Grayson Fm., Waco Dam, Texas, USA; group of zooids with ovicells in an early flustrine anascan. C, Dacryoporella gutta (Lang), NHMUK D44443, Coniacian, Chalk, Chatham, Kent, UK; single zooid from a uniserial colony of this early hippothoomorph ascophoran. D, Pelmatopora marsupitorum Lang, NHMUK D41056, Campanian, Chalk, Rottingdean–Saltdean, Sussex, UK; cribrimorph ascophoran (Infraorder Acanthostega) showing autozooids with characteristic frontal shields consisting of overarching, laterally and medially fused spines, and surrounded by small avicularia and elongate kenozooids (e.g. arrows). E, unidentified cribrimorph ascophoran, NHMUK BZ7735, Campanian, Needs Camp, East London, South Africa; reduced spinocystal frontal shield becoming overgrown (arrows) by the outer walls of encircling kenozooids. F, Bathosella sp., NHMUK BZ7736, details as for E; umbonulomorph ascophoran showing frontal shields ringed by areolar pores. Scale bars represent 500 lm (A, D, F); 200 lm (B–C); 100 lm (E)." Figure and caption from from Taylor and Waeschenback (2015) in Palaeontology under the Permissions of The Palaeontological Association.
As discussed by Taylor and Waeschenbach (2015), the evolution and eventual rise to prominence by the cheilostomes can be considered as a three-phase process (see figure below). For more than 50 million years, cheilostomes were represented by only a handful of genera. In the second phase, these early cheilostomes diversified rapidly until reaching peak Mezosoic diversity at the end of the Cretaceous. This diversification coincided with a large increase in morphological variation. Though they declined during the Cretaceous-Paleogene mass extinction, cheilostomes fared substantially better than the stenolaemate cyclostomes and surpassed their Mesozoic diversity in the early Cenozoic. Since that time, the third phase of cheilostomes diversity has been defined by relatively constant diversity.
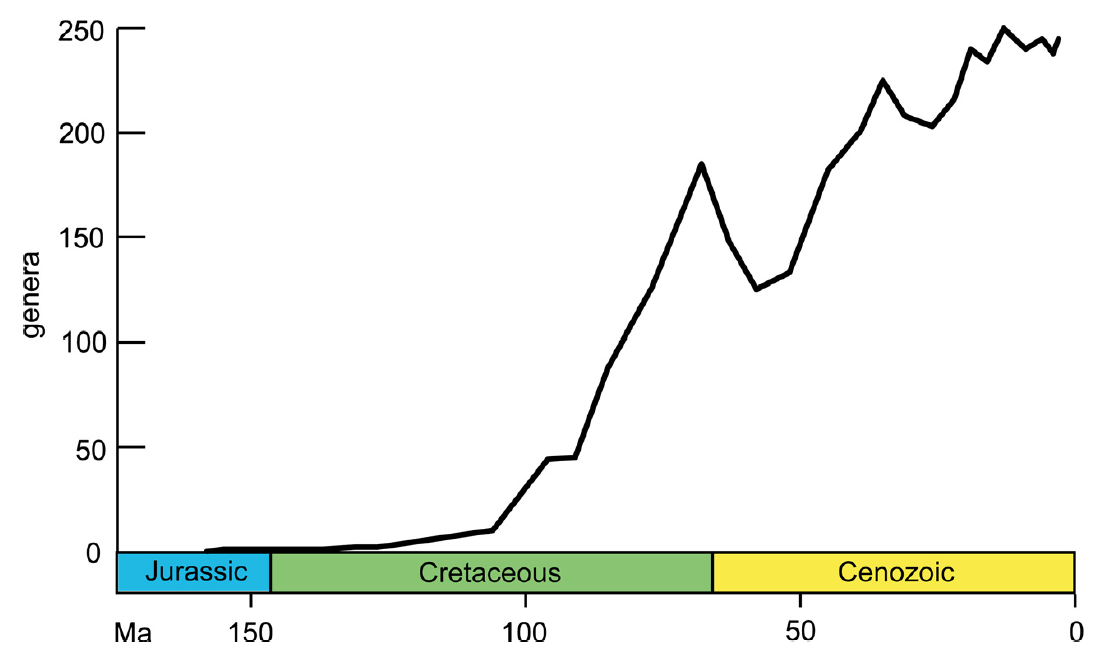
"Generic diversity of cheilostome bryozoans based on stage level data published by Ostrovsky et al. (2008) for the pre-Albian, and McKinney and Taylor (2001) for the Albian–Pleistocene. Cheilostome diversity remained low until the Cenomanian, increased rapidly through the Late Cretaceous, peaking in the Maastrichtian, declined at the KT boundary and at the end of the Danian, and increased again through most of the Palaeogene." Figure and caption from Taylor and Waeschenback (2015) in Palaeontology under the Permissions of The Palaeontological Association.
Two evolutionary innovations are commonly considered to have led to the diversification of the Cheilostomata. The first innovation was larval brooding. Rather than broadcast spawning, the cheilostomes evolved ovicells to house their larvae as the larvae developed. In effect, this may have reduced gene flow between different populations, as it would have substantially reduced the distance over which larval cheilostomes dispersed. Though this isolation may have facilitated a faster rate of speciation, several groups of cheilostomes with brooding larvae never reached high diversity. Thus, brooding alone may not be sufficient to explain the Cretaceous diversification. The second major innovation was an increased defensive capacity, as mentioned above. This increased defensiveness included calcified frontal shields; polymorphic zooids capable of forming jaw-like, snapping structures; and a whip-like vibracula to wipe predators and epibionts away from the colony (check out a pair of vibricula in the video below). Given that the Mesozoic was a time of increasingly intense predation in the marine realm, these features likely contributed to the diversification and longevity of the Cheilostomata.
Check out the vibricula in this video. In the bottom middle of the image, two thin vibricula can be seen extending beyond the lophophores. "Bryozoan with lophophores and a pair of vibracula" by Katie Quinn. Source: YouTube.
Class Phylactolaemata
Phylactolaemata are exclusively freshwater bryozoans and can be found in lakes, ponds, streams, rivers, and estuaries. Today, this group is represented by approximately 70 species, which belong to five families, all within a single order, Plumatellida. Though it has been speculated that phylactolaemates may have been the first bryozoans to evolve, there is no evidence of this group in the fossil record until the Permian, some 200 million years after the first stenolaemate appeared in the record. The absence of phylactolaemates from the fossil record is due in large part to their lack of skeletal hard parts. Unlike the stenolaemates and gymnolaemates that produce hard calcium carbonate skeletons, phylactolaemates produce only an unmineralized, gelatinous material to form their colonies.
Check out a living freshwater bryozoan in this video, "What is this Brain-Like Blob Found in a Lagoon?" by National Geographic. Source: YouTube.
The first appearance of phylactolaemates in the fossil record, and essentially all subsequent occurrences, comes in the form of a statoblast—a durable mass of cells produced from which new colonies can develop. Statoblasts, which are unique to phylactolaemates, are a strategy for asexual reproduction and dispersal. Like similar structures produced by other freshwater invertebrates (e.g., the water flea, Daphnia), statoblasts can also serve as an insurance policy against changing conditions. When they are fully developed, statoblasts have a hard, two-valved chitinous shell that can protect the individual for extended periods of time. Some statoblasts drop off from their colony and get buried in the bottom sediments, others fill with air and float away, and others remain attached to their colony. If the colony dies and environmental conditions eventually improve (e.g., winter ends and a lake thaws), or the statoblast arrives in a new location suitable for growth, statoblasts can form new colonies to replace their original colony or populate a new area. Whereas colonies of some species may produce only one statoblast, others have been reported to produce more than 800,000 of these asexual reproductive structures. At the end of the video above, you can see hundreds of tiny statoblasts on the hand of the person holding the colony.

Statoblasts of the phylactolaemate bryozoan, Cristatella mucedo. Image by Lamiot; Creative Commons Attribution-Share Alike 4.0 International license.
This freshwater group is also distinct from the Stenolaemata and Gymnolaemata in that its colonies are composed entirely of autozooids—non-specialized individuals whose purpose is to feed and excrete waste—without any more specialized zooid forms. Though all three classes of bryozoans employ a hermaphroditic strategy, phylactolaemates are again unique in that they are simultaneous rather than sequential hermaphrodites, like the majority of marine bryozoans. Thus, in addition to reproducing asexually through statoblasts, phylactolaemates also reproduce sexually. To do so, sperm is released from individual zooids into the water, where it circulates about the colony. Other zooids capture the sperm, much like capturing food particles with their lophophores, and use this to fertilize their eggs. Through their unique, and relatively primitive form, the Phylactolaemata likely provide a closer resemblance to the ancestral bryozoans—that potentially lived in the Cambrian, or earlier—than do the more specialized Stenolaemata and Gymnolaemata.
References and further reading
Di Martino, E., P. D. Taylor, and R. W. Portell. 2019. Anomia-associated bryozoans from the upper Pliocene (Piacenzian) lower Tamiami Formation of Florida, USA. Palaeontologica Electronica, 22: 1 - 56.
Cheetham, A. H. 1986. Branching, biomechanics and bryozoan evolution. Proceedings of the Royal society of London. Series B. Biological Sciences, 228: 151-171.
Cheetham, A. H. 1986. Tempo of evolution in a Neogene bryozoan: rates of morphologic change within and across species boundaries. Paleobiology, 12: 190-202.
Harvell, C. D. 1984. Predator-induced defense in a marine bryozoan. Science, 224: 1357-1359.
Jackson, J. B., and A. H. Cheetham. 1990. Evolutionary significance of morphospecies: a test with cheilostome Bryozoa. Science, 248: 579-583.
Mckinney, F. K., P. D. Taylor, and S. Lidgard. 2003. Predation on bryozoans and its reflection in the fossil record. In Predator—Prey Interactions in the Fossil Record (pp. 239-261). Springer, Boston.
O'Dea, A., and B. Okamura. 1999. Influence of seasonal variation in temperature, salinity and food availability on module size and colony growth of the estuarine bryozoan Conopeum seurati. Marine Biology, 135: 581-588.
O'Dea, A., and B. Okamura. 2000. Intracolony variation in zooid size in cheilostome bryozoans as a new technique for investigating palaeoseasonality. Palaeogeography, Palaeoclimatology, Palaeoecology, 162: 319-332.
O'Dea, A., and J. Jackson. 2009. Environmental change drove macroevolution in cupuladriid bryozoans. Proceedings of the Royal Society B: Biological Sciences, 276: 3629-3634.
Pagès-Escolà, M., Bock, P. E., D. P. Gordon, S. Wilson, C. Linares, B. Hereu, and M. J. Costello. 2020. Progress in the discovery of extant and fossil bryozoans. Marine Ecology Progress Series, 635: 71-79.
Taylor, P. D., B. Berning, and M. A. Wilson. 2013. Reinterpretation of the Cambrian ‘bryozoan'Pywackia as an octocoral. Journal of Paleontology, 87: 984-990.
Taylor, P. D., and A. Waeschenbach. 2015. Phylogeny and diversification of bryozoans. Palaeontology, 58: 585-599.
Telford, M. J., G. E. Budd, and H. Philippe. 2015. Phylogenomic insights into animal evolution. Current Biology, 25: R876-R887.
Usage
Unless otherwise indicated, the written and visual content on this page is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 International License. This page was written by Jansen A. Smith. See captions of individual images for attributions. See original source material for licenses associated with video and/or 3D model content.




