Chapter contents:
Cnidaria
– 1. Anthozoa
–– 1.1 Scleractinia ←
–– 1.2 Rugosa
–– 1.3 Tabulata
–– 1.4 Octocorallia
– 2. Hydrozoa
– 3. Cubozoa
– 4. Scyphozoa
This page is by Jonathan R. Hendricks and was last updated on November 1, 2019.
A Virtual Collection of 3D models of scleractinian corals may be accessed here.
Above: close-up views of a variety of solitary and colonial coral exoskeletons.
Overview
The anthozoan order Scleractinia includes the "true corals" or "stony corals," which are represented today by about 1500 extant species. Scleractinians first appeared in the early Middle Triassic and have been the dominant (though not exclusive) reef-building organisms over the past 240 million years.
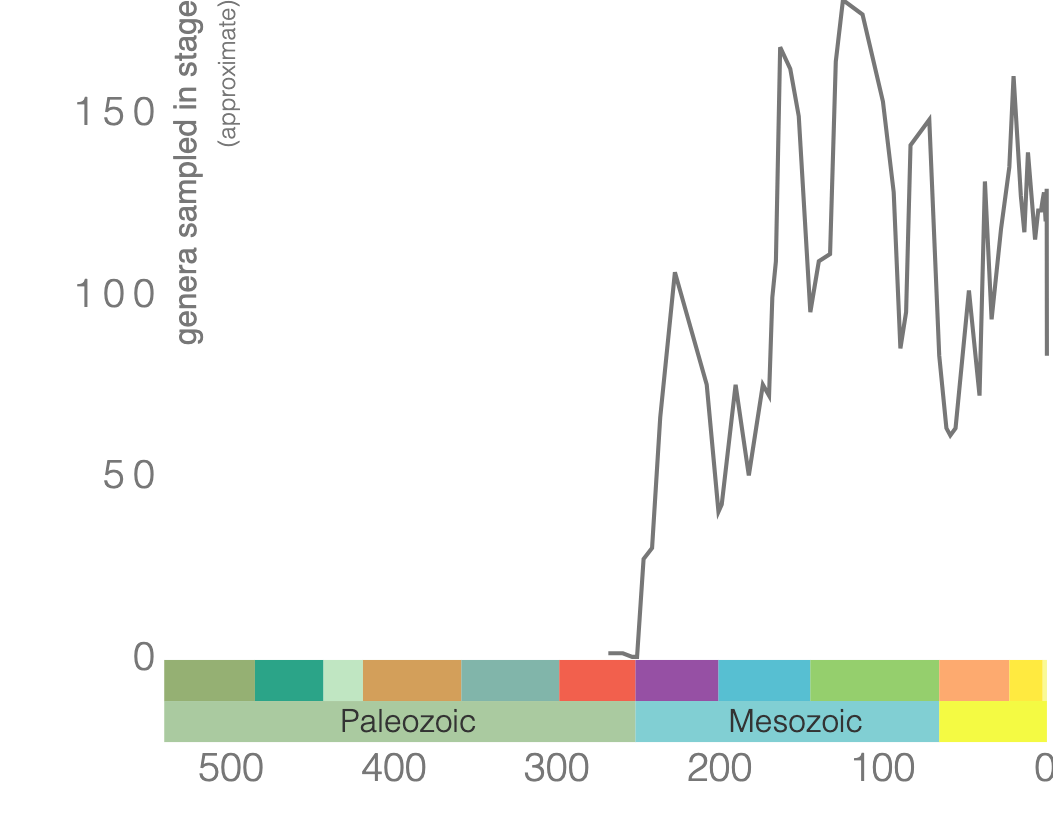
Genus-level diversity of scleractinian corals since the Triassic. Image created using the Paleobiology Database Navigator.
The reefs built by modern scleractinian corals are Earth's most biodiverse marine ecosystems and provide numerous valuable services to human societies. Scleractinian corals may be either solitary or colonial in form and always have skeletons composed of the aragonite form of calcium carbonate. All species, past and present, are marine and occupy habitats ranging from shallow to deep water. All colonial, reef-building species receive energy from symbiotic algae called zooxanthellae.
Morphology of Scleractinian Corals
At a very basic level, scleractinian corals can be sorted morphologically based on whether they live as solitary individuals (single polyps), or as colonies comprised of many individual polyps. The polyps themselves resemble miniature sea anemones (scleractinian corals and sea anemones are both anthozoans and are very closely related). The tentacles of the polyps are covered with nematocysts that are used to capture prey. As the polyp grows, it builds its aragonite skeleton. The polyps of colonial species occupy only the top-most portion of the mature skeleton, the vast majority of which is non-living.

Polyp of the solitary coral Flabellum sp.; compare with the exoskeleton of extant Flabellum moseleyi shown further below. Image by Nick Hobgood (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license).

Polyps of the colonial scleractinian coral Montastraea cavernosa, which lives in the Caribbean. This specimen is on exhibit at the Museum of the Earth, Ithaca, New York. Image by Jonathan R. Hendricks.
Like Paleozoic rugose corals (and some tabulate corals), the skeletons of scleractinian corals have radial structures called septa. In scleractinian corals, these are arranged in multiples of six. Six or 12 primary septa are deposited first. Secondary septa are then inserted between the primary septa, and this is followed again by later insertion of tertiary (3rd order) and quaternary (4th order) septa. This is illustrated below in the modern coral Flabellum.
Morphology of solitary coral exoskeletons
The exoskeletons of solitary corals are often small in size and cup shaped. The entire exoskeleton (occupied by a single polyp) is called a corallum. The outer wall of the corallum is called the epitheca. Extensions of the radially-aranged septa through the epiteca are called costae (not always present). A pillar-like central structure called the columella may be present or absent.
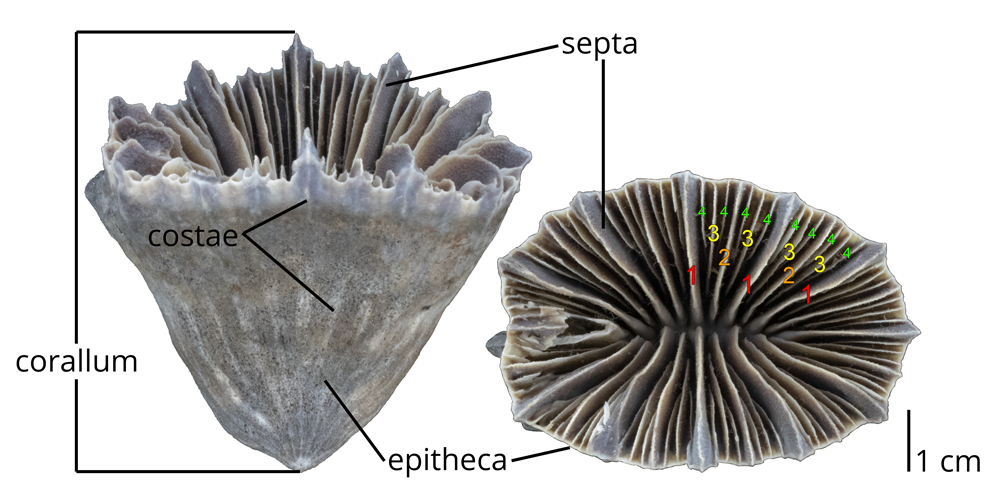
The extant solitary coral Flabellum moseleyi (Family Flabellidae) collected at a depth of over 800 ft from the southwest coast of Florida (PRI 76895). Numbers indicate primary (1), secondary (2), tertiary (3), and (4) quaternary septa (this species has 12 primary septa). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Compare with image above of a live specimen of Flabellum. Image by Jonathan R. Hendricks.
Morphology of colonial coral exoskeletons
The skeletons of colonial corals exhibit a much greater diversity of forms than those of solitary species. Often, their growth forms are dependent upon the habitats in which they live. Robust, rounded colonies are often favored in high-energy habitats with lots of wave action, while delicate branching forms are usually associated with quieter environments.
An entire colonial coral colony is known as a corallum; the space occupied by a single polyp within the colony is called a corallite. Individual corallites have their own radial septa, which often attach to a centralized pillar called the columella.
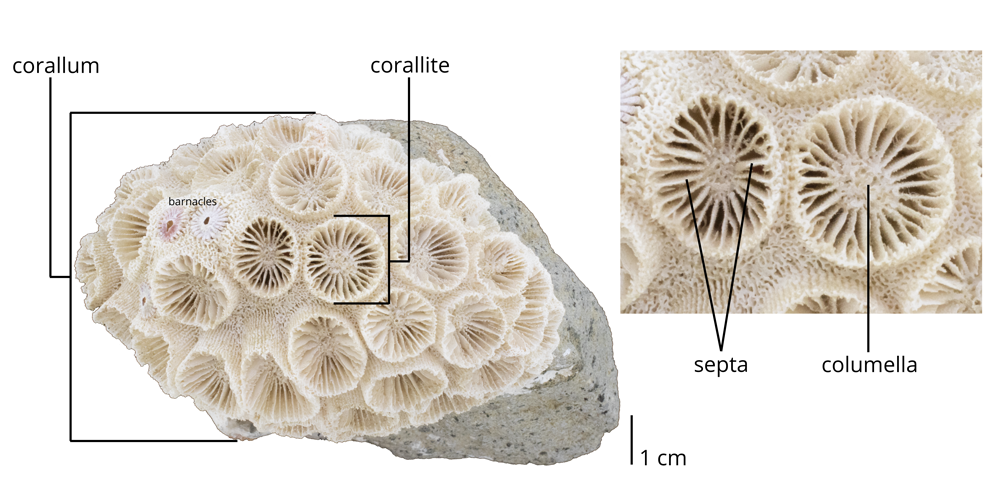
Fossil specimen of the colonial scleractinian coral Astrangia sp. from the Late Pleistocene Ft. Thompson Fm. of Hillsborough County, Florida (PRI 76863). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Image by Jonathan R. Hendricks. See 3D model of this specimen below.a
Zooxanthellate vs. Azooxanthellate Corals
Many corals have developed a remarkable symbiotic relationship with microscopic dinoflagellate algae that invade and then live inside of their tissues. These photosynthetic algae belong to the genus Symbiodinium and are more commonly called zooxanthellae (pronounced “zo-zan-thel-ae”). The zooxanthellae provide the coral animal with extra energy and increased calcification rates, which fuels rapid growth. In exchange for providing this energy boost, the zooxanthellae receive protection within the stinging coral’s tissues, as well as a ready supply of critical nutrients that are produced by the coral animal.
Because zooxanthellae depend upon sunlight for photosynthesis, the corals that harbor them (called zooxanthellate corals) need to live in clear, shallow water (the photic zone, up to 70 m depth). The colonial scleractinian species that build modern reefs are all zooxanthellate. There are around 800 living species of zooxanthellate corals (Kitahara et al., 2016).
As their name implies, azooxanthellate corals lack symbiotic algae (the prefix a- often means “without”). Because they don’t have photosynthetic algae to keep happy, they commonly live at much greater depths than zooxanthellate species (up to 6000 m!), though some species also live in shallow water. In contrast to zooxanthellate corals, most of the 700 living azooxanthellate species are solitary (Kitahara et al., 2016).
Importance and Distribution of Modern Coral Reefs
Importance
Coral reefs are the most biologically diverse marine habitats and are created by colonial, zooxanthellate scleractinians. Large reefs can support thousands of different species of animals. One reason why they are so biodiverse is that their three-dimensional complexity provides a large number of ecological niches for different kinds of organisms to occupy.

Living scleractinian corals on the Great Barrier Reef, Queensland, Australia. Image by Toby Hudson (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license).
Besides being home to a great diversity of species, coral reefs also provide substantial value to people as fishing grounds, locations for recreation and tourism, and as physical barriers that protect coastal structures from wave energy; it is estimated that their annual value to the United States alone is $3.4 billion (NOAA). Learn more about the importance of coral reefs to human society here.

Coral reefs provide substantial value to human communities. Image from NOAA (public domain).
Distribution
Modern scleractinian coral reefs have a global distribution, but are relatively restricted in terms of the conditions where they can live.
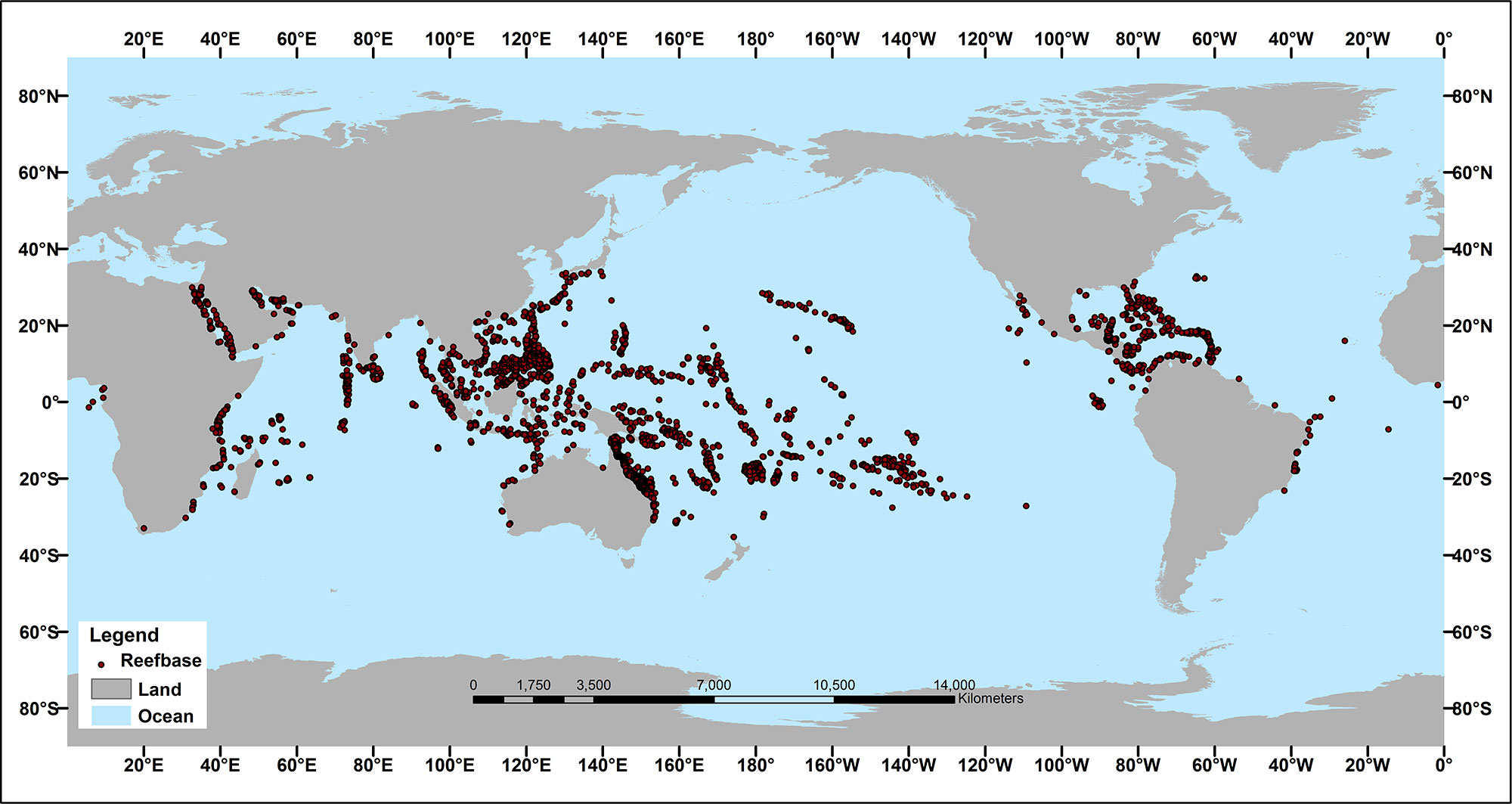
Map showing the modern distribution of scleractinian coral reefs around the world. Image by NOAA (public domain).
Three of the major factors that control the distributions of modern coral reefs are:
- Water temperature
- Nutrient levels
- Water depth
Compare the map above, which shows the distribution of modern coral reefs, with the maps below that illustrate each of these three factors.
Water Temperature
For the most part, corals reefs are found in warm, tropical waters that range from 25–29° Celsius (77–84° Fahrenheit).
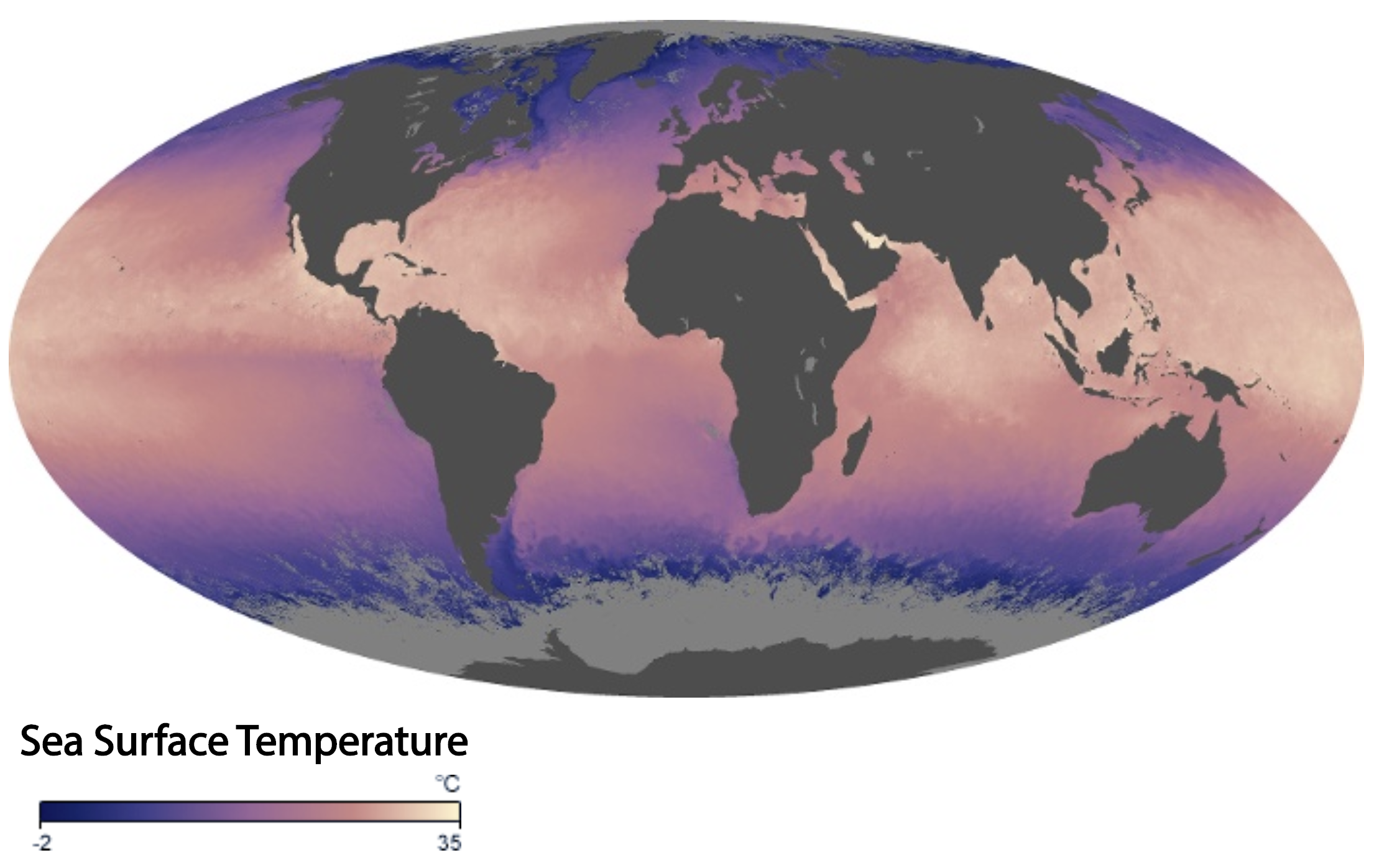
Global sea surface temperature during September 2019. Image source: NASA (public domain).
Nutrient Levels
Reef-building scleractinian corals prefer areas that are low in nutrients. One reason for this is that high nutrient levels can greatly accelerate the growth of algae that can very quickly cover and overwhelm corals. The map below shows concentrations of chlorophyll in ocean water, which is commonly used as a proxy for nutrient levels: higher nutrient levels correspond with great amounts of photosynthesizing microorganisms in the water column (phytoplankton).
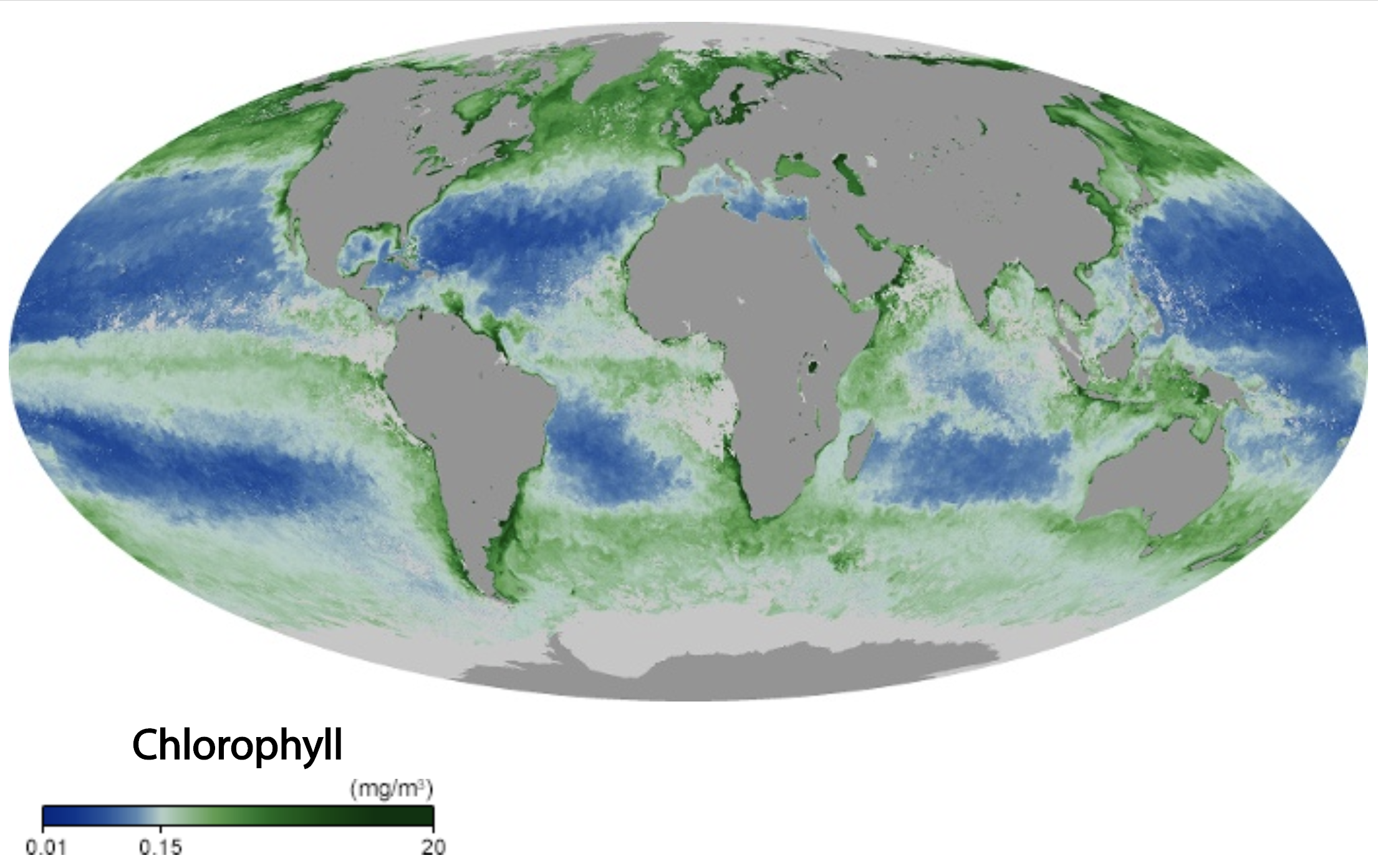
Global marine cholorophyll levels during September 2019. Image source: NASA (public domain).
Nutrient levels tend to be highest in coastal areas with significant upwelling caused by ocean currents, near river deltas (which carry nutrients from land to the ocean), and in areas near human civilization (caused by agricultural runoff and other sources of pollution).
Water Depth
Because of their symbiotic photosynthesizing zooxanthellae, reef-building corals can only live within the uppermost photic zone (up to ~70 m depth) and are therefore restricted to shallow-water environments.
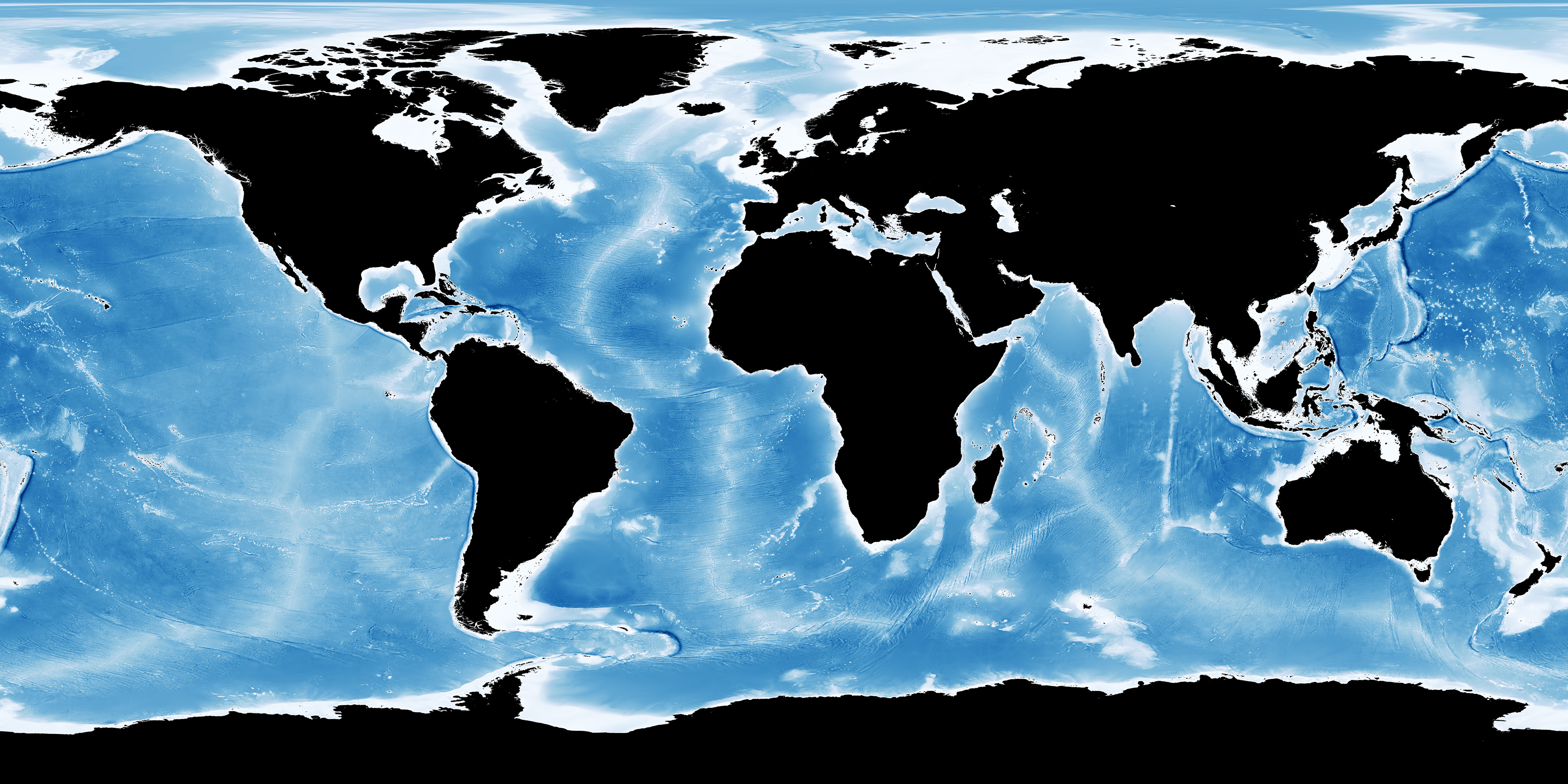
Global ocean water depth. White and light blue shading indicates shallow water; darker blues indicate deep water. Image source: NASA (public domain).
Coral Bleaching
Living, healthy zooxanthellate corals are usually brownish in color. Instead of being a sign of good health, bright, white-colored coral is often a sign of coral that is distressed because it has lost its brownish-colored zooxanthellae. This is called coral bleaching: the coral animal itself is mostly transparent, so the underlying white aragonite skeleton of the coral becomes visible when the brown-colored algae are no longer present.

The scleractinian coral Acropora (Keppel Islands, Great Barrier Reef, Australia). The white-colored colony in the foreground has been bleached, while the brownish-colored colony in the background is healthy. Image by "Acropora" (Wikimedia Commons; Creative Commons Attribution 3.0 License).
"Coral Bleaching" by Bozeman Science (YouTube).
Even though a number of factors can cause corals to bleach—including stress, pollution, and changes in water salinity—the most important factor at regional and global scales is temperature. Zooxanthellate corals are very picky about water temperature and most only thrive in ocean water that is between 25–29 degrees Celsius (77–84 degrees Fahrenheit).
Global climate change is causing marine water temperature to increase, frequently above levels that are tolerable to corals. A common response of zooxanthellate corals to increased water temperatures is the expulsion of the symbiotic algae from their tissues, causing bleaching. For details about why this might happen at the cellular scale, see the recent review by Oakley and Davy (2018). Reef-building corals can survive short amounts of time without their zooxanthellae, but the prolonged absence of these symbionts is usually fatal.
Mass coral bleaching events are becoming more and more common around the world as ocean temperatures rise, and have resulted in the loss nearly a quarter of Earth's coral reefs over the past several decades (NOAA). Given their numerous benefits to biodiversity and human society, the potential loss of coral reef ecosystems is one of the most significant challenges presented by global climate change.
Scleractinian Coral Phylogeny and Classification
One might predict that a phylogeny of scleractinian corals would show two major clades: one composed of colonial, zooxanthellate species, the other composed of solitary, azooxanthellate corals. Perhaps surprisingly, this is not the case at all!
Recent molecular work has shown that species within the same taxonomic family sometimes vary in whether they are colonial or solitary, as well as whether or not they are symbiotic with zooxanthellae. As summarized by the authors of such a recent study, this “suggests that coloniality and symbiosis in corals either evolved multiple times or that they were lost more than once” (Barbeitos et al., 2010).
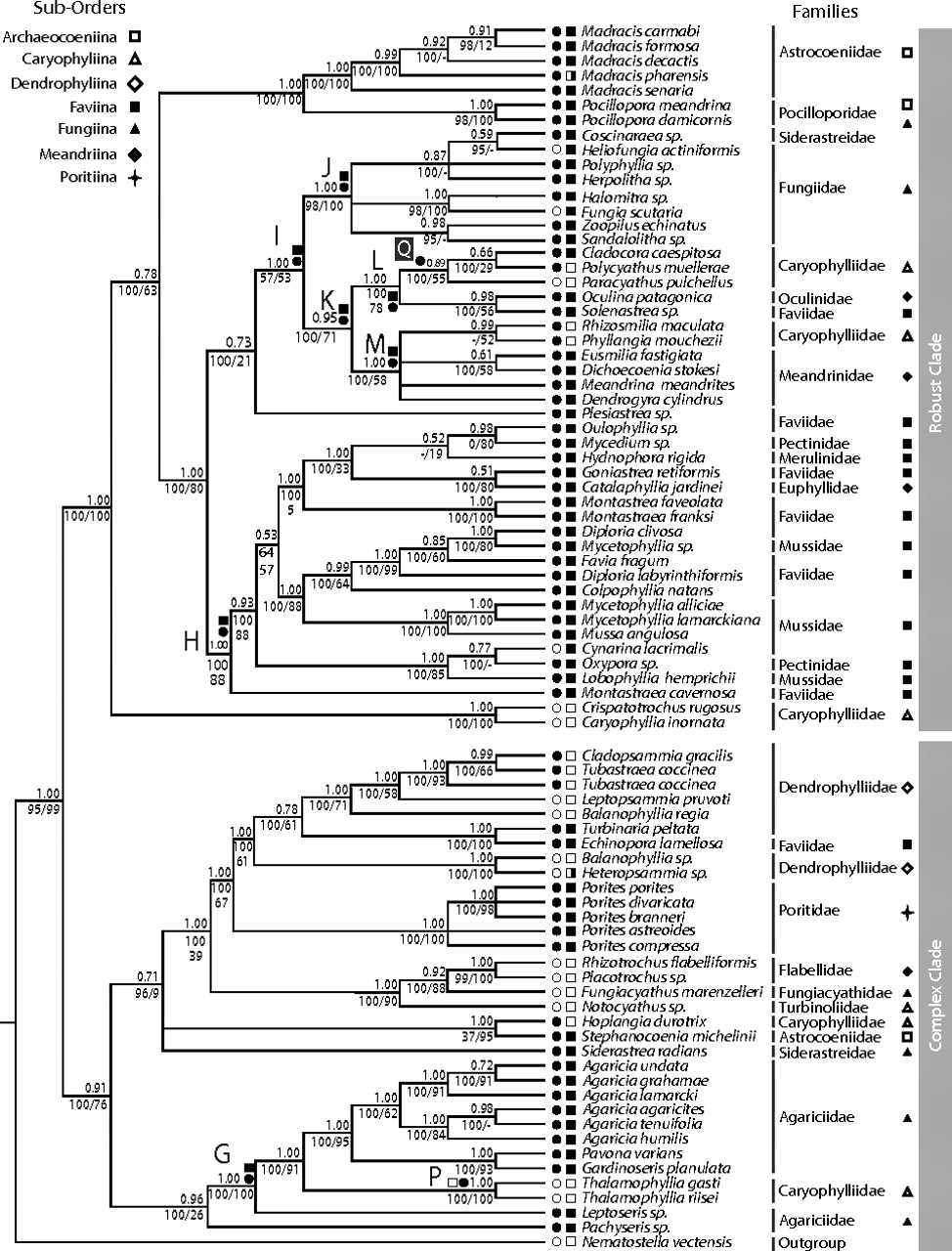
Molecular phylogeny of extant scleractinian corals published by Barbeitos et al. (2010, Fig. 1). Filled circles are colonial species; open circles are solitary species; filled squares are zooxanthellate species; open squares are azooxanthellate species. This figure was published in Proceedings of the National Academy of Sciences (PNAS) 107(26): 11877-11882; copyright retained by article authors and used here as per PNAS policy.
Evidence from the molecular phylogenetic analysis of Barbeitos et al. (2010) suggests that all of the azooxanthellate lineages that they analyzed descended from multiple zooxanthellate ancestors. Further, their phylogeny demonstrates that solitary coral lineages descended from at least six different colonial ancestors. These results are suggestive that the earliest scleractinian corals were colonial, harbored symbiotic algae, and almost certainly lived in shallow water in the photic zone. If true, this means that solitary, azooxanthellate forms have independently originated multiple times in the history of scleractinian coral evolution and have also moved to deeper habitats. Losing their symbiotic algae presented a significant cost (loss of energy available for increased growth rates), but also allowed these corals to occupy a far greater range of habitats—especially beyond the photic zone)—a trait that is known to buffer against extinction risk.
A very important lesson from recent molecular phylogenetic analyses of scleractinian corals is that the morphological characteristics that have been traditionally used to sort them into major taxonomic subgroupings are highly unreliable. For example, note in the phylogenetic tree above that species traditionally assigned to the solitary, azooxanthellate family Caryophylliidae based on major morphological features are actually located in five different clades! This means that the morphological similarity that was used to assign them to Caryophylliidae is likely a result of evolutionary convergence, not common ancestry. Instead of focusing upon macroscopic features of coral form, Barbeitos et al. (2010) suggested that coral biologists and paleontologists should instead concentrate on characterization of microscopic features of the coral skeleton in order to find characters that might have phylogenetic significance, using the tree of relationships suggested by the independent molecular dataset as a backbone for searching for similar features shared by closely-related species with overall forms (e.g., colonial vs. solitary) that might be very different.
The 1500 living species of scleractinian corals are divided among 31 families and approximately 240 genera (Kitahara et al., 2016). Recent molecular phylogenetic analyses divide these into three major clades that are informally known as the “basal,” “complex,” and “robust” clades (the complex and robust clades are sister taxa). Importantly, there is essentially no morphological evidence that supports these major clades, though they are supported by nearly all genetic data. At lower taxonomic levels, many of the families traditionally established by morphological (mostly skeletal) data have also been determined by analysis of molecular data to be polyphyletic (e.g., Fukami et al, 2008). While macroscopic features of the coral skeleton are increasingly being viewed as highly unreliable for classification, Kitahara et al. (2016) note that “microstructural characters … have proven to be phylogenetically informative at the family level” (p. 51) and are also less prone to environmental influence than macroscopic features. Thus, the way forward for recognizing the phylogenetic associations of extinct corals will be to study their micromorphological and microstructural features and compare them with similar features in extant taxa of known phylogenetic position (see additional discussion by Budd et al., 2010).
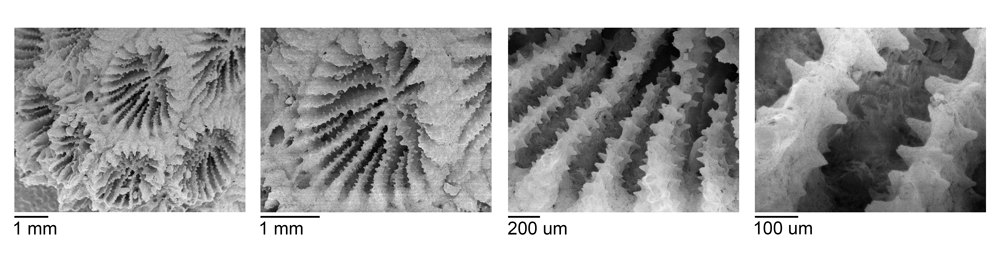
Scanning electron micrographs (SEMs) of the micromorphological details of corallites of an unidentified coral from the Plio-Pleistocene of Sarasota County, Florida. Note the fine teeth on the septa.
A sampling of fossil and modern scleractinian diversity, organized by taxonomic family, is presented below.
Examples of Solitary Scleractinian Corals
Family Flabellidae

The Miocene solitary coral Flabellum appendiculatum from Antwerp, Belgium (PRI 44626). Specimen is from the research collections of the Paleontological Research Institution. A 3D model of this specimen is shown below. Image by Jonathan R. Hendricks.
Fossil specimen of the solitary coral Flabellum appendiculatum from the Miocene of Belgium (PRI 44626). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 3.5 cm. Model by Emily Hauf.
Family Caryophylliidae

Fossil specimen of the solitary coral Placocyathus variabilis from the Plio-Pleistocene of Lee County, Florida (PRI 76896). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Image by Jonathan R. Hendricks.
Family Faviidae
Fossil specimen of the solitary scleractinian coral Thysanus excentricus from the Pliocene Tamiami Formation (Pinecrest Beds) of Lee County, Florida (PRI 76870). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 14 cm. Model by Emily Hauf.
Fossil specimen of the solitary scleractinian coral Scolymia lacera associated with a oyster that appears to have grown upon the coral (PRI 76871). Specimen is from the Plio-Pleistocene of Sarasota County, Florida and is part of the research collection of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 11 cm. Model by Emily Hauf.
Fossil specimen of the solitary scleractinian coral Scolymia lacera from the Plio-Pleistocene of Hendry County, Florida (PRI 76872). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Maximum diameter of specimen is approximately 6.5 cm. Model by Emily Hauf.
Examples of Colonial Scleractinian Corals
Family Rhizangiidae
Fossil scleractinian coral Astrangia sp. with associated Ceratoconcha and Balanus barnacles (PRI 76863). Specimen is from the Pleistocene Fort Thompson Formation of Hillsborough County, Florida. Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 12 cm. Model by Emily Hauf.
A worn fossil specimen of the bivalve Mercenaria mercenaria that has been extensively bored into by the sponge Clionia sulphurea and encrusted by the colonial scleractinian coral Astrangia danae. Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Length of shell is approximately 8.5 cm. Model by Emily Hauf.
Fossil specimen of the colonial scleractinian coral Septastrea crassa from the Pliocene Tamiami Fm. (Pinecrest Beds) of Sarasota, Florida (PRI 45562). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 23.5 cm. Model by Emily Hauf.

Fossil specimen of the colonial, encrusting scleractinian coral Septastrea marylandica from the Plio-Pleistocene of Sarasota County, Florida (UF 9229). Specimen is from the collections of the Florida Museum of Natural History. Learn more about this species on the Neogene Atlas of Ancient Life.

Scanning electron micrograph (SEM) of two corallites of fossil scleractinian coral Septastrea marylandica from the Plio-Pleistocene of Sarasota County, Florida. Learn more about this species on the Neogene Atlas of Ancient Life.
Fossil coral Septastrea marylandica from the upper Pliocene Tamiami Fm. (Pinecrest Beds) of Sarasota County, Florida (PRI 70753). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Maximum length of specimen is 46.0 mm. This species lived by encrusting and growing on the shells of other organisms, in this case a snail shell. Learn more about this species on the Neogene Atlas of Ancient Life. Model created by Emily Hauf.
Family Meandrinidae

Fossil specimen of the colonial scleractinian coral Dichocoenia stokesii from the Plio-Pleistocene of Lee County, Florida (PRI 76892). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Image by Jonathan R. Hendricks.
Fossil colonial scleractinian coral Meandrina meandrites from the Pliocene Tamiami Formation of Lee County, Florida (PRI 76875). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Maximum diameter of specimen is approximately 22 cm. Model by Emily Hauf.
Modern specimen of the sclearactinian coral Eusmilia fastigata from Sand Key Reef, Florida Keys, Florida (PRI 76767). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 13.5 cm. Model by Emily Hauf.
Family Siderastreidae

Stacked fossil specimens of the colonial scleractinian coral Siderastrea pliocenica from the Plio-Pleistocene of Lee County, Florida (PRI 76859). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Also see 3D model of this specimen below. Image by Jonathan R. Hendricks.
Fossil scleractinian coral Siderastrea pliocenica from the Pliocene Tamiami Formation of Lee County, Florida (PRI 76859). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 14 cm. Model by Emily Hauf.
Fossil specimen of the scleractinian coral Siderastrea pliocenica from the Plio-Pleistocene of Sarasota County, Florida (PRI 76873). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Maximum diameter of specimen is approximately 6.5 cm. Model by Emily Hauf.
Fossil specimen of the scleractinian coral Siderastrea dalli, with the coral Dichocoenia eminens growing upon it; also present are the coral barnacles Ceratoconcha prefloridana, as well as other small encrusters. Specimen is from the Pleistocene Caloosahatchee Formation of Florida (PRI 76789). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 12 cm. Model by Emily Hauf.

Holocene specimen of the extant colonial coral Siderastrea siderea from the Caribbean (PRI 76893). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Image by Jonathan R. Hendricks.
Family Oculinidae

Fossil specimen of the colonial scleractinian coral Oculina sarasotana from the Plio-Pleistocene of Lee County, Florida (PRI 76894). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Image by Jonathan R. Hendricks.
Family Faviidae
Fossil colonial scleractinian coral Pseudodiploria clivosa from the Pleistocene Miami Oolite of Monroe County, Florida (PRI 76861). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 9 cm. Model by Emily Hauf.
Fossil specimen of the scleractinian coral Pseudodiploria strigosa from the Pliocene Tamiami Fm. (Pinecrest Beds) of Lee County, Florida (PRI 76868). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 12 cm. Model by Emily Hauf.

Fossil specimen of the colonial coral Manicina areolata from the Pleistocene of Collier County, Florida (UF 107704). Specimen is from the collections of the Florida Museum of Natural History. Learn more about this species on the Neogene Atlas of Ancient Life.
Fossil specimen of the scleractinian coral Manicina areolata from the Pleistocene Caloosahatchee Fm. of Lee County, Florida (PRI 76869). Specimen is from the research collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 13.5 cm. Model by Emily Hauf.
Family Agariciidae
Modern specimen of the colonial scleractinian coral Agaricia agaricites from the Florida Keys (PRI 76794). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 10 cm. Model by Emily Hauf.
Family Montastraeidae
Modern specimen of the colonial scleractinian coral Montastraea cavernosa from off Monroe County, Florida (PRI 13355). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Maximum dimension of specimen is approximately 11 cm. Model by Emily Hauf.
Family Not Established
Modern specimen of the colonial scleractinian coral Cladocora arbusucula from near St. Petersburg, Pinellas County, Florida (PRI 13376). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Maximum diameter of specimen is approximately 7.5 cm. Model by Emily Hauf.

Fossil specimen of the colonial scleractinian coral Solenastrea hyades from the Early Pleistocene Caloosahatchee Fm. of Charlotte County, Florida (UF 12239). Specimen is from the collections of the Florida Museum of Natural History. Learn more about this species on the Neogene Atlas of Ancient Life.
References and Further Reading
Barbeitos, M.S., S.L. Romano, and H.R. Lasker. 2010. Repeated loss of coloniality and symbiosis in scleractinian corals. Proceedings of the National Academy of Science (PNAS) 107(26): 11877-11882.
Budd, A. F., S. L. Romano, N. D. Smith, and M. S. Barbeitos. 2010. Rethinking the phylogeny of scleractinian corals: a review of morphological and molecular data. Integrative & Comparative Biology 50(3): 411-427.
Fukami, H., C. A. Chen, A. F. Budd, A. Collins, C. Wallace, Y.-Y. Chuang, C. Chen, C.-F. Dai, K. Iwao, C. Sheppard, and N. Knowlton. 2008. Mitochondrial and nuclear genes suggest that stony corals are monophylletic but most families of stony corals are not (order Scleractinia, class Anthozoa, phylum Cnidaria). PLoS ONE 3(9): e3222.
Kitahara, M.V., H. Fukami, F. Benzoni, and D. Huang. 2016. The new systematics of Scleractinia: integrating molecular and morphological evidence. Pp. 41-60 in S. Goffredo and Z. Dubinsky (eds.), The Cnidaria, Past, Present, and Future: The World of Medusa and Her Sisters, Springer International Publishing Switzerland.
Lough, J.M, and M.J.H. van Oppen. 2018. Introduction: coral bleaching—patterns, processes, causes and consequences. Pp. 1-8 in M.J.H. van Oppen and J. M. Lough (eds.), Coral Bleaching, Ecological Studies 233, Springer International Publishing AG.
National Oceanic and Atmospheric Administration. 2019. Fast Facts: Coral Reefs.
Oakley, C.A., and S. K. Davy. 2018. Cell biology of coral bleaching. Pp. 189-211 in M.J.H. van Oppen and J. M. Lough (eds.), Coral Bleaching, Ecological Studies 233, Springer International Publishing AG.



