Chapter by:
Jaleigh Q. Pier, Paleontological Research Institution, Ithaca, New York
This chapter was first publicly shared on December 5th, 2019; last updated on September 16, 2020.
Chapter citation:
J.Q. Pier. 2019. Porifera. In: The Digital Encyclopedia of Ancient Life. https://digitalatlasofancientlife.org/learn/porifera
Chapter contents:
Porifera ←
–– 1. Archaeocyatha
–– 2. Stromatoporoidea
–– 3. Demospongiae
–– 4. Hexactinellida
–– 5. Calcarea
–– 6. Homoscleromorpha
Associated materials:
Virtual Teaching Collection of 3D photogrammetry models of Porifera fossils available here!
Above image: Fossil porifera specimens from the collection at the Paleontological Research Institution, Ithaca NY. Image by Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Phylum Porifera Snapshot
- Living classes: Demospongiae, Hexactinellida, Calcarea, Homoscleromorpha
- Extinct classes: Archaeocyatha, Stromatoporoidea
- Diversity: ~9235 extant species
- Ecology: sessile filter feeders
- Key features of group: multicellular, asymmetrical, flagellated choanocyte cells, porous structure made of spongin and spicules
- Fossil Record: Precambrian to Recent
Overview
Sponges make up the simplest animal group on the planet: phylum Porifera (from the Latin porus ‘pore’ and ferre ‘to bear’). Having no digestive tract, localized sensory region, or true tissues, they are little more than a cluster of cells supported by a structure of spongin and spicules. Spongin is the flexible material that makes up the body wall of the sponge, while spicules are hard, spiny secretions that help to provide a reinforced structure. The cells lining the inside of the sponge are called choanocytes (‘collar cells’), which have a ‘tail-like’ flagellum. The beating flagella of the choanocytes create water currents that flow through the canals and pores in the sponge, bringing in oxygen and particles of food that are consumed.
Sponges encompass a vast diversity of asymmetrical shapes, sizes, and even hardnesses (ranging from soft and flexible to rock-hard). They have a global distribution, occupy shallow to deep water conditions, and occur in virtually all marine and freshwater environments. In this chapter we will explore the diversity and fossil record of phylum Porifera.
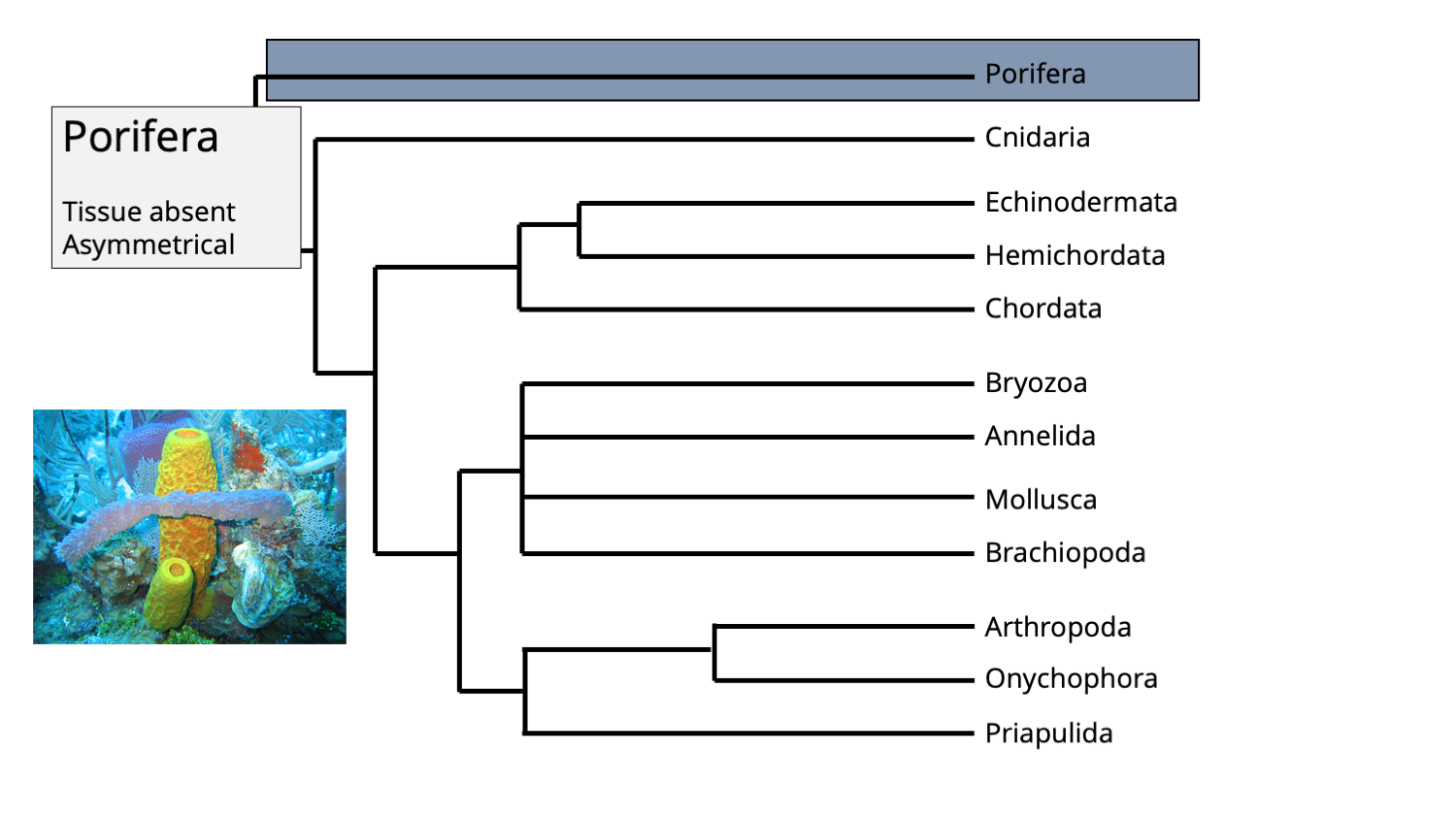
The position of phylum Porifera in the animal tree of life. Phylogenetic tree by Jonathan R. Hendricks; inset image of modern sponges by NOAA (Creative Commons; public domain).
Phylogeny
Being the oldest animal group, sponges have had the longest opportunity to diversify and evolve, which makes their phylogeny rather complex to sort out. Contrary to some early molecular work that suggested that sponges might be paraphyletic, there is a growing consensus that sponges represent a monophyletic group, with the Silicea (a clade that includes the sister taxa Demospongiae and Hexactinellida) sharing a common ancestor with Calcarea and Homoscleromorpha. However, this is continuously under debate and you should consult the recent literature to determine what standing is most current.
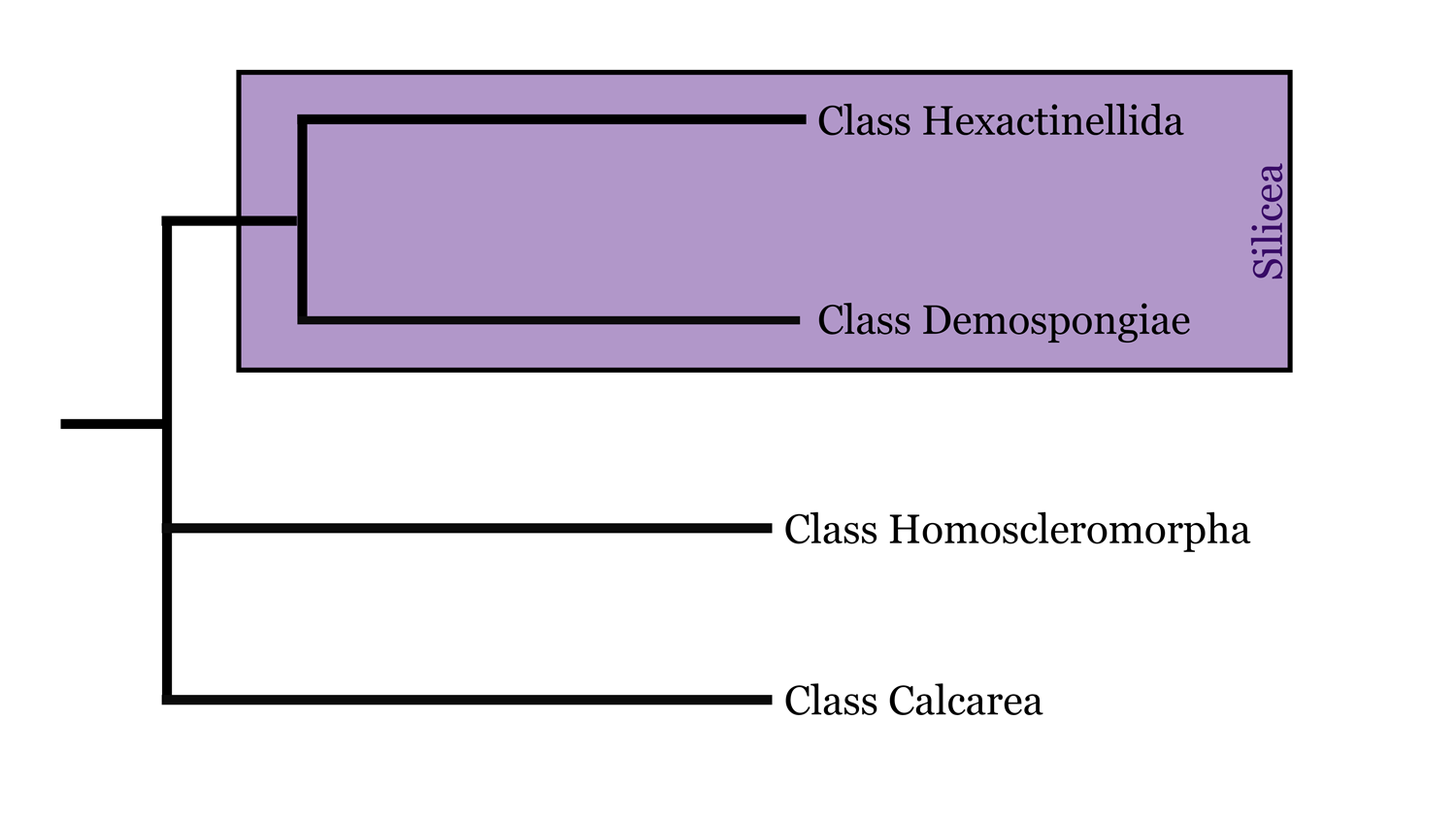
Highly simplified overview of porifera phylogeny based in part on the hypothesis of relationships presented by Botting and Muir (2018). Image by: Jaleigh Q. Pier, licensed under a Creative Commons Attribution-Share Alike 4.0 International License.
General Features of Sponges:
Sponges are morphologically simple and their basic features are labeled in the image below. Although most of these features are characteristics of all classes, some have become specialized for certain environments.
If you were to pick up a kitchen or bath sponge, you would feel its soft flexible structure. This soft texture corresponds with the spongin of a living sponge. Although modern kitchen and bath sponges are now made of synthetic materials, living sponges were once collected for human use. The holes throughout the sponge are called ostia, which help channel water flow (containing of food particles) through the sponge. The large openings at the top of a sponge are called oscula, which expels the filtered water and waste out of the sponge.
- Osculum: (oscula, plural) large opening at the top of the sponge where water is expelled
- Ostia: pores in the body wall
- Spongin: a flexible protein that makes the soft ‘body’ of the sponge
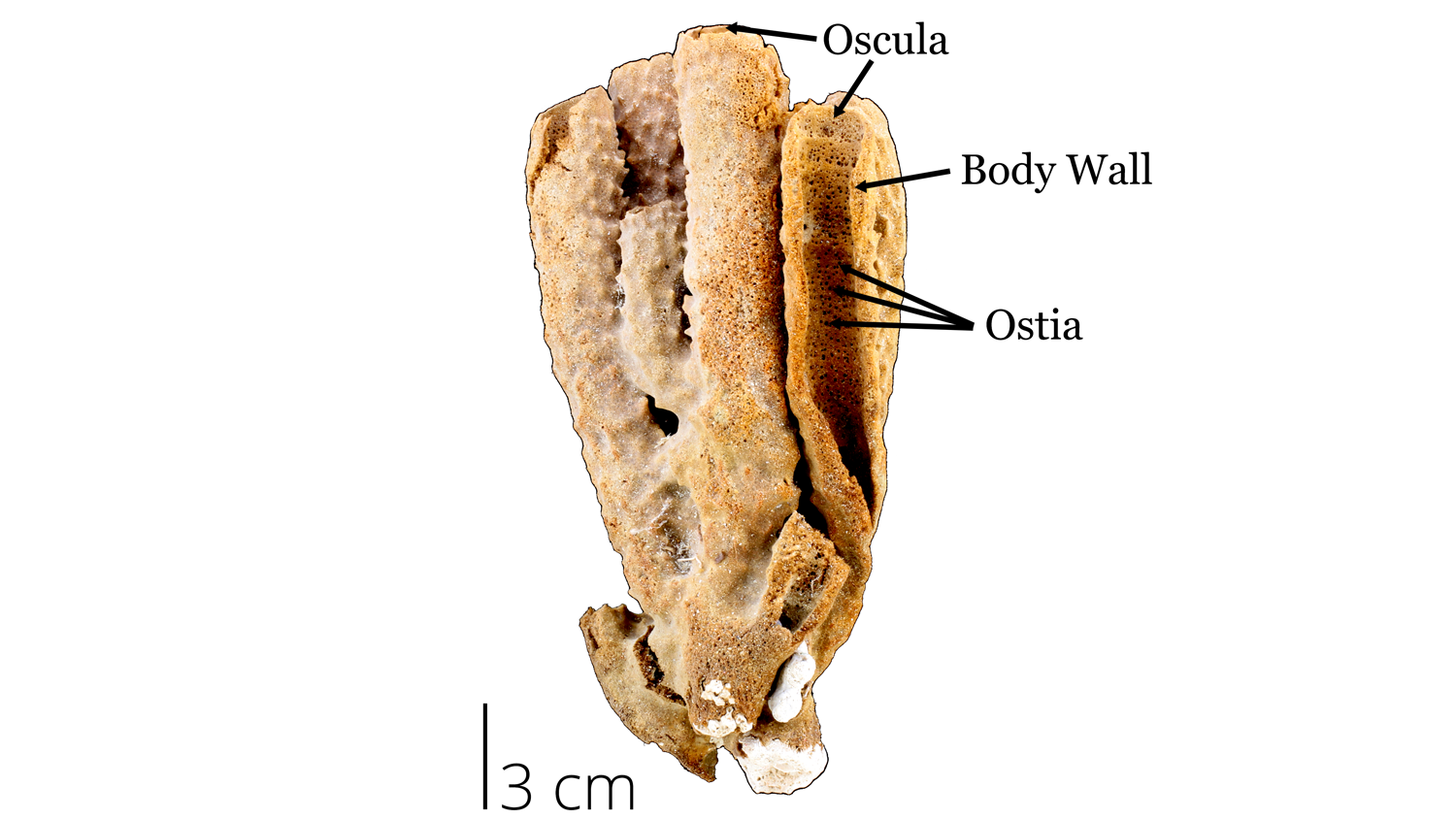
Recent demosponge from the collections of the Paleontological Research Institution, Ithaca NY. Image by Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.

Recent demosponge attached to a scallop shell from the collections of the Paleontological Research Institution, Ithaca NY. Image by Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Sponges have developed several body plans of increasing complexity. Ascon grade is the simplest form of pores connecting to a choanocyte-lined internal chamber. Sponges of intermediate complexity are of sycon grade. Although the basic structure is still the same, choanocytes line several incurrent canals, creating more surface area for filter feeding and generation of stronger currents. The most complex structure is leucon grade, where thick walls house an intricate network of canals connecting several flagellated chambers. Although modern calcareous sponges are the only living groups with ascon and sycon grades, they are common fossil forms. The diagram below distinguishes these different structural types.
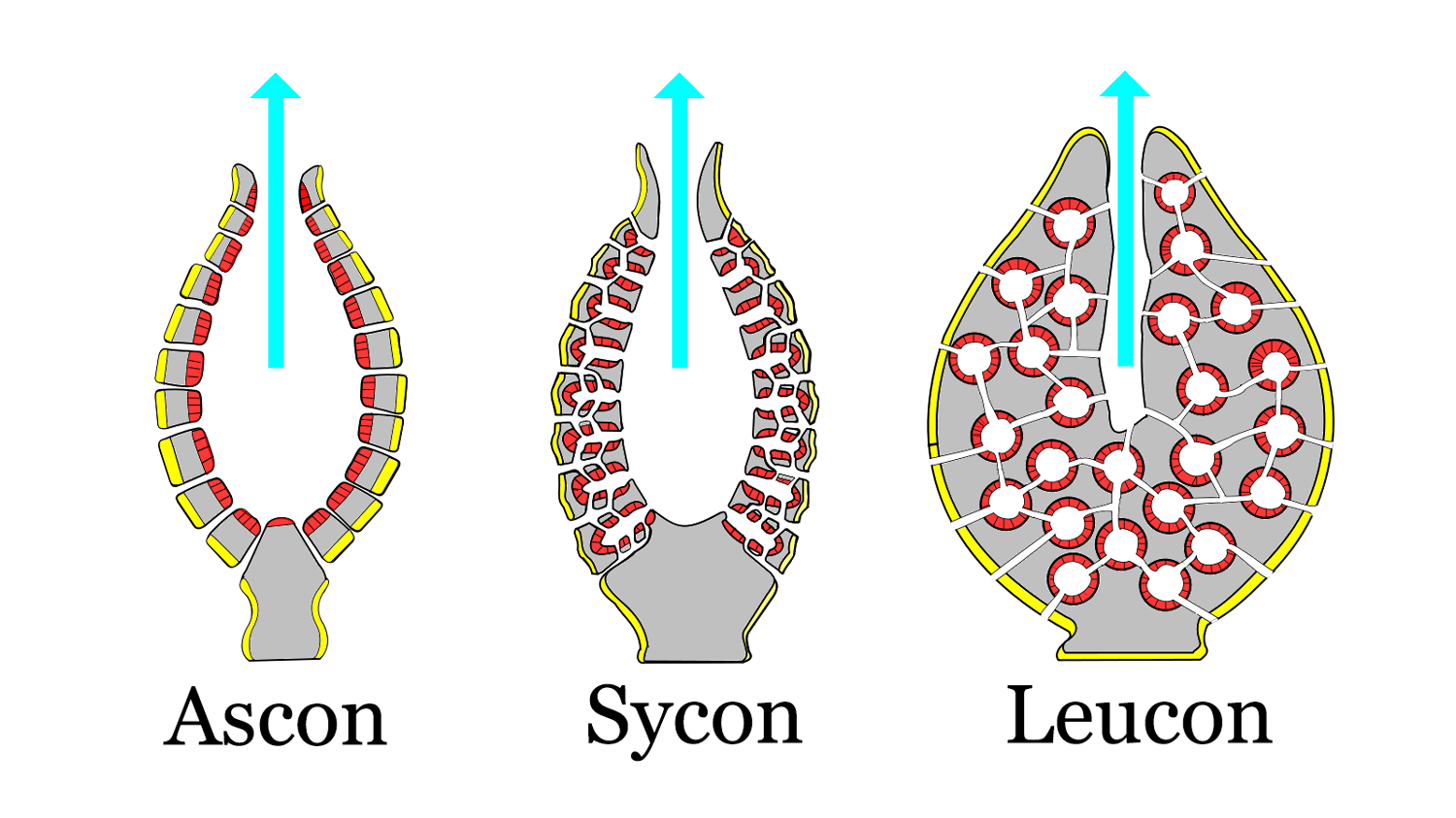
Sponge body plans of increasing complexity. The red areas are lined with choanocytes, the yellow areas are lined with pinacocytes. Modified from original image by 'Philcha' (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Public Domain Dedication).
Sponges also have several microscopic features that are more difficult to see with the naked eye. Most cells in a sponge are totipotent, meaning they can move around and change function throughout their lifetime. They essentially act as stem cells and switch to different forms when needed. Sponges have several kinds of specialized cells that carry out specific life tasks. Check out the video below to see different sponge cells in action!
- Archaeocytes: amoeboid cells that ingest/digest food particles and transport nutrients to other cells
- Choanocytes: or ‘collar cells’ are flagellated cells in the body wall, which create currents to capture food particles
- Pinacocytes: cells that create an outer covering of the sponge, like ‘skin’
- Sclerocytes: cells that secrete spicules
- Spongocytes: cells that secrete spongin
- Mesohyl: cell layer between the choanocytes and external/internal body wall of sponge, this is where skeletal elements are produced and gametes are stored by many sponge cell types
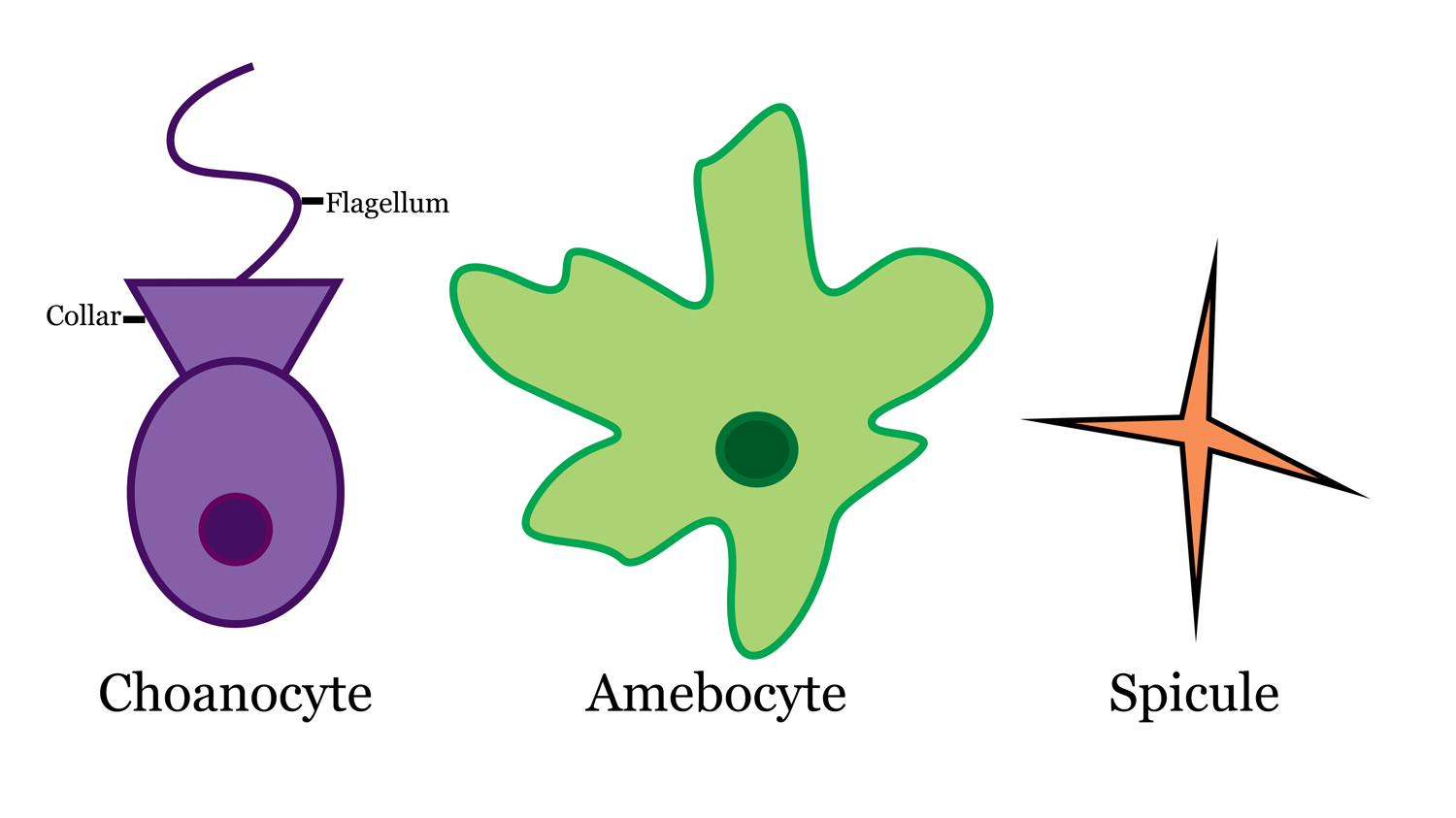
Sponge cells and spicule. Image by: Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
"Sponge Animation: Wild Ride Through a Sponge" by Shape of Life (shapeoflife.org)
To be classified as a sponge, an organism must have spicules, which are needle-like secretions of calcium carbonate or silica. Spicules are classified based on their structural characteristics and size. Megascleres are large, whereas microscleres are small. The number of axes in a spicule differentiates between being called a monoaxon (one axis), triaxon (three axes), tetraxon (four axes), or polyaxon (many axes). If you think of a plus sign (+), it has two axes; one horizontal and one vertical. The number of crossing lines in a spicule determines how many axes it will have. Spicules can also be described in terms of the number of directions in which they grow. Each pointed end of a spicule signifies a single growth direction, which differntiates between monoactine, diactine, triactine, hexactine, tetracline, and polyactine.
For example, a toothpick-shaped spicule has only one line (axis) so it would be monoaxon. Since it has two distinct points (growth directions), it would also be called diactine. Some examples of these are shown below.
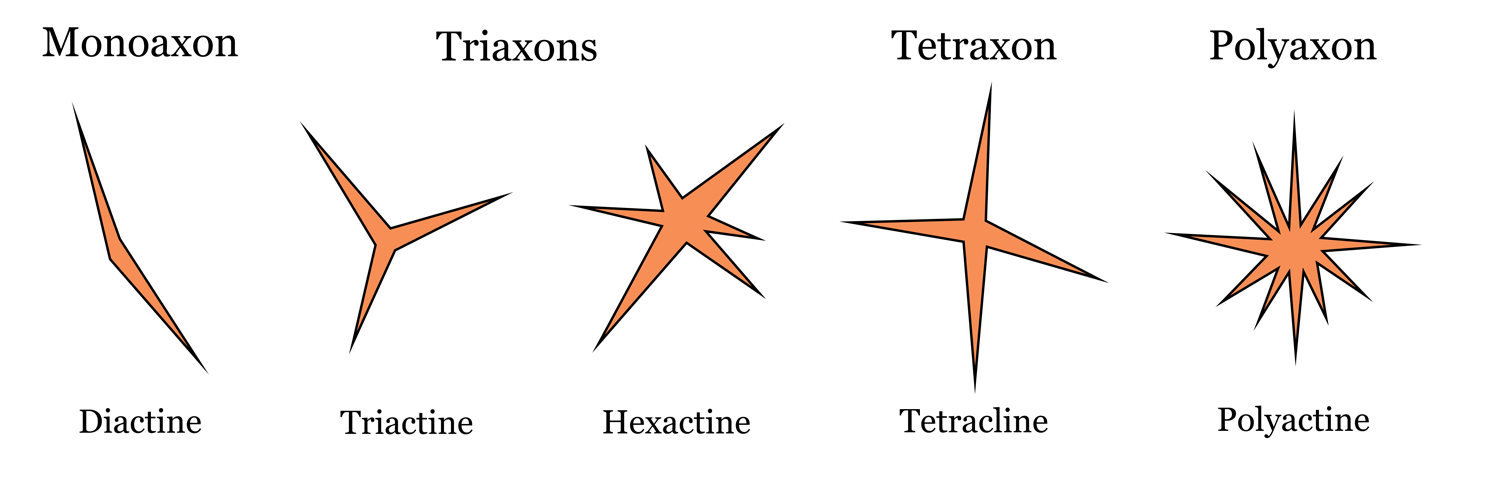
Sponge spicule diversity. Top labels correspond to number of axes, bottom labels correspond to number of growth directions. Image by: Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
The Amazing Sponge
At first sight, sponges may seem like stationary, boring creatures. They have, however, several unsuspecting qualities. Only a few of them will be described here.
Ocean Filters
Sponges may have been the first ever ecosystem engineers, meaning they drastically changed the environment around them in a beneficial way. Being such efficient filters, they cleanse the water around them and help cycle nutrients through the water for other living organisms to take advantage of.
J. Keith Rigby explains “…a black loggerhead sponge, 50cm in diameter and 30cm tall, may draw approximately 1000 L of water through its canal systems in a single day. Other sponges may pass 10,000 to 20,000 times as much water as their volume through their canals in a single day” (Boardman, Cheetham, and Rowell, 1987, p. 132). Watch how this happens in the video below!
"Amazing footage of sponges pumping!" by BlueWorldTV (YouTube).
Regeneration
Sponges also have the ability to regenerate into their original form if broken or separated into pieces. If different sponge species are dissociated into cellular pieces, they will come back together only with cells of their own kind. Check out this process in the video below!
An excerpt from the 'First Life' documentary narrated by David Attenborough by BBC (YouTube).
Cure for Cancer?
When researchers were studying the regenerative properties of sponges, they also noticed other properties of the cell cultures that could be used in developmental biology and immunology fields. Several chemical compounds have been discovered in sponges that have potential implications for the treatment in humans of pain, inflammation, and diseases such as cancer and Alzheimer’s (Munro et al. 1999).
"Prescription Oceans: Part 1" by ChangingSeasTV (YouTube).
Spawning
Sponges can reproduce in two ways: asexual reproduction via budding off from a piece of their body, or through sexual reproduction via spawning to combine eggs and sperm. Being sessile creatures, sponges are stuck in place until reproduction when their larvae can disperse and swim to new locations. Some species even brood their larvae within their body wall, protecting them from predators.
Giant barrel sponges spawning during a full moon by 'MENCHIBUS' (YouTube).
Symbiosis
Many sponges are symbiotic, meaning they have physically close, long-term relationships with other organisms. These include many microorganisms such as bacteria, microalgae, archaea, cyanobacteria, and fungi. Some species can house bacteria in their mesohyl, which eat other potentially infectious microorganisms and other things that could make the sponge sick. In deep sea environments other symbionts may be used for chemosynthesis to provide extra nutrition and energy (Hentschel et al., 2002).
Sponges are a source of protection and housing for a variety of larger organisms including chordates such as fish and many invertebrates. One species of crab that is informally known as the ‘sponge crab’ will hold a piece of sponge on its back until it attaches to provide extra camouflage. Watch the video below to see one in action!
A sponge crab by Debbie Lawrence Carter (YouTube).
Fossil Record
Studying the early evolution of multicellular animals has been a focal point of research for biologists and paleontologists alike. Sponges are important for understanding this transition. Their primitive nature lacks most distinguishing animal features and their choanocyte cells resemble several features of single-celled eukaryotes, possibly indicating the transition from a cell colony to a multicellular organism. Choanocytes essentially act as stem cells for sponges in that they provide essential life functions, such as acquiring food, but also create the ability to reproduce through gamete production. New developments in gene sequencing and DNA extraction are consistently illuminating our knowledge of how these transitions evolved and further developed.
Although the oldest fossil evidence for sponges is firmly rooted in the Cambrian, other evidence suggests an even earlier existence. Possible sponge body fossils has been proposed from the Ediacaran, although poor preservation suggest prevents definitive identifications. Other evidence of biomarkers and loose spicules date further back to the Cryogenian (720-635 million years ago), but without additional evidence, it’s difficult to say if these came from early sponges or rather related single-celled organisms. Watch the video below to learn more about these discoveries!
"This Simple Sea Creature Might Have Been Earth's First Animal" by Newsy Science (YouTube).
Early stem-group sponges from the Paleozoic are commonly lumped together and called 'Reticulosans'. These sponges have thin walls with hexactine spicules, and many taxa have been placed in this ‘trash bin’ group (meaning that their placement within sponge phylogeny is uncertain). The ambiguous structure of early sponges and poor preservation has made sorting out their early evolution a very difficult process.
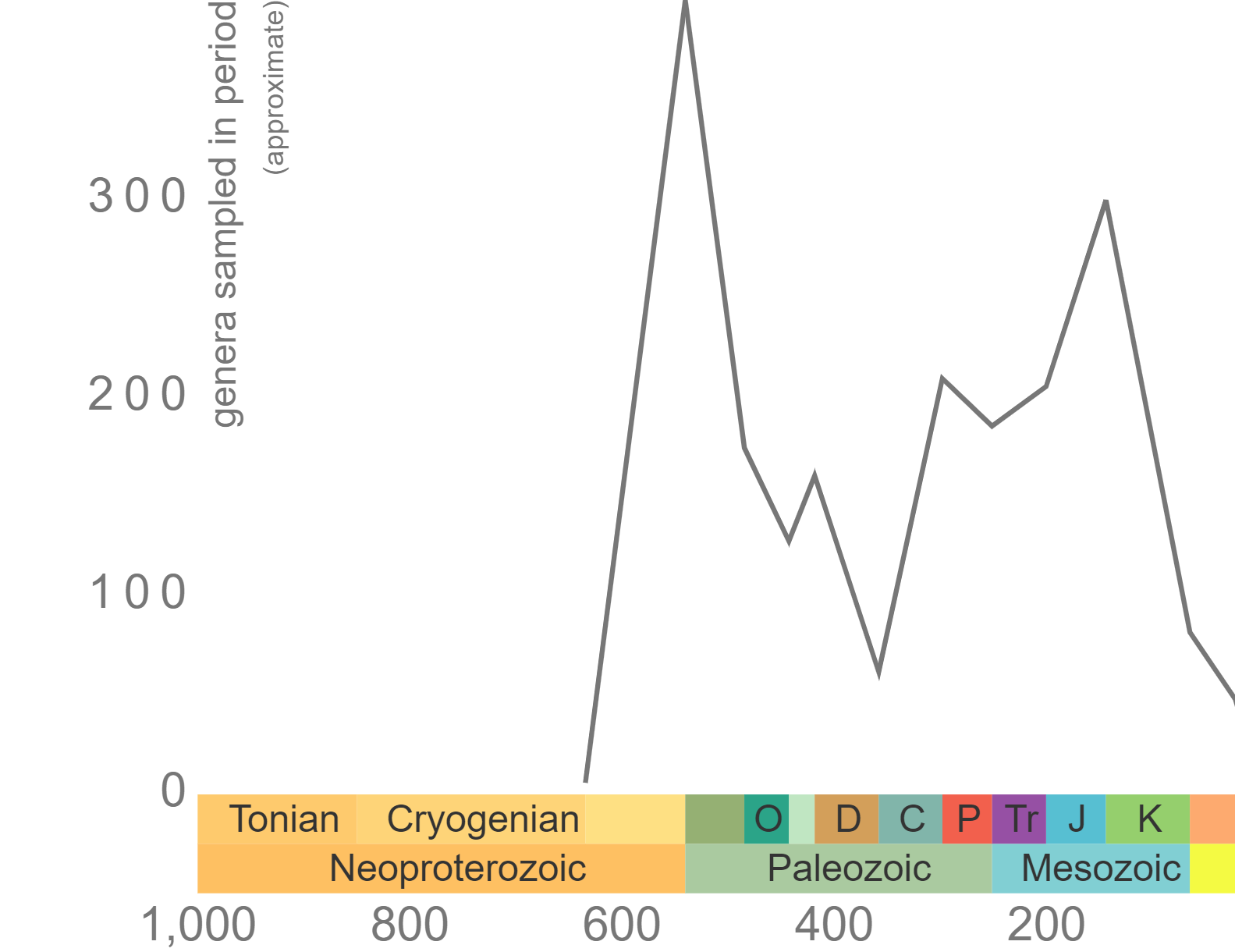
Phanerozoic genus-level diversity of "Porifera" (graph generated using the Paleobiology Database Navigator).
Sponges often become disarticulated soon after death, with only spicules remaining. Therefore, most of the fossil record of non-mineralizing sponges consists of their spicules. There are, however, some body fossils of non-mineralized sponges that are known from lagerstatten deposits (a couple of examples are shown below).

Fossil specimen of Chancelloria pentacta, a possible early sponge from the Middle Cambrian. Specimen from the collections of the Paleontological Research Institution in Ithaca, NY. Image by Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.

Fossil specimen of Vauxia gracilinta, a possible early sponge from the Middle Cambrian. Specimen from the collections of the Paleontological Research Institution in Ithaca, NY. Image by Jaleigh Q. Pier is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Soon after the Cambrian explosion, evidence for early members of at least three sponge classes arose. Below is a chronological list of when major sponge groups originated, which you can read more about in the following sections.
- Archaeocyatha, a cryptic sponge clade, became major reef builders during their brief existence in the Early Cambrian. You can learn more about them in the next section!
- Demospongiae, Hexactinellida, and Calcarea, three of the current sponge classes, all evolved within the Cambrian period.
- Stromatoporoids arose during the Ordovician and were the second group (after Archaeocyatha) to form reefs; they were especially important reef builders during the Silurian and Devonian, when they reached their peak diversity.
- Most recently, class Homoscleromorpha developed during the Mesozoic, although there may be some evidence from the Carboniferous.
References and further reading
Adamska, M. 2016. Sponges as models to study emergence of complex animals. Current Opinion in Genetics & Development: 39, pp. 21-28.
Boardman, R.S., Cheetham, A.H., and Rowell, A.J. 1987. Fossil Invertebrates. Blackwell Scientific Publications. 713 pp.
Botting, J.P, and Muir, L.A. 2018. Early sponge evolution: A review and phylogenetic framework. Palaeoworld: 27, pp. 1-29.
Brusca, R.C., and G.J. Brusca. 2002. Invertebrates Second Edition. Sinauer Associates Inc. Publishers, Sunderland MA. 936 pp.
Chang, S. et al. 2019. The Ediacaran-Cambrian rise of siliceous sponges and development of modern oceanic ecosystems. Precambrian Research: 333, pp. 1-16.
Dembowska, W.S. 1926. Study on the Habits of the Crab Dromia vulgaris M.E. The Biological Bulletin: 50(2), pp. 163-178.
Hentschel, U et al. 2002. Molecular evidence for a uniform microbial community in sponges from different oceans. Applied and Environmental Microbiology 68: 4431-4440.
Hogg, et al. 2010. Deep-sea sponge grounds: Reservoirs of biodiversity. UNEP-WCMC Biodiversity Series No. 32. UNEP-WCMC, Cambridge, UK.
Manconi, R. and R. Pronzato, 2015. Chapter 8 Phylum Porifera. In Thorp, J.H., and Rogers, C. editors: Ecology and General Biology (Fourth Edition): Elsevier: Amsterdam, pp. 133-157.
Munro, M.H., Blunt, J.W., Dumdei, E.J., Hickford, S.J., Lill, R.E., Li, S., Battershill, C.N., & Duckworth, A.R. (1999). The discovery and development of marine compounds with pharmaceutical potential. Journal of biotechnology, 70 1-3, 15-25 .
Pomponi, S.A. 2006. Biology of the Porifera: cell culture. Canadian Journal of Zoology: 84, pp. 167-174.
Rigby, J.K. 1969. Sponges and reef and related facies through time. In Rigby, J.K., editor. Reefs through time, Part J. North American Paleontological Convention, Chicago. Lawrence, KS: Allen Press; 1971. pp. 1374-1388.
Van Soest, R.W.M., et al. 2012. Global Diversity of Sponges (Porifera). PLoS ONE: 7(4), pp. 1-23.
Wörheide, G et al. 2012. Deep Phylogeny and Evolution of Sponges (Phylum Porifera). In Becerro, M.A., Uriz, M.J., Maldonado, M. and Turon, X. editors: Advances in Marine Biology: 61, The Netherlands: Amsterdam, Academic Press, pp. 1-78.
Yin, Z. et al. 2015. Sponge grade body fossil with cellular resolution dating 60 Myr before the Cambrian. PNAS: 112(12), pp. 1-7.
Usage

Unless otherwise indicated, the written and visual content on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This page was written by Jaleigh Q. Pier. See captions of individual images for attributions. See original source material for licenses associated with video and/or 3D model content.



