Chapter by:
Jonathan R. Hendricks, Paleontological Research Institution, Ithaca, New York
This partial chapter was first publicly announced on May 29, 2020.
Chapter citation:
Hendricks, J. R. 2020. Phylum Chordata. In: The Digital Encyclopedia of Ancient Life.
Chapter contents:
Chordata: Overview and Basal Taxa ←
- Jawless Vertebrates
- Note: Additional pages are in preparation.
Topics covered on this page: Chordate Phylogeny; Cephalochordates; Tunicates; and Stem Vertebrates.
Virtual Collection: Available here.
Image at top: Lectotype of Pikaia gracilens from the Middle Cambrian Burgess Shale Member of the Stephen Fm., British Columbia (USNM PAL 57628). Image retrieved from iDigBio; copyright Smithsonian Institution.
Introduction
The phylum Chordata includes many familiar animals. Indeed, most of the animals on exhibit at any zoo are chordates. So are most of the animals featured on nature programs on television. Major subgroups of chordates include "fishes" (a paraphyletic grouping; see below), amphibians (frogs, toads, and salmanders), "reptiles" (another paraphyletic group), birds, and mammals. If you have a pet at home other than a scorpion, tarantula, or snail, it is likely a chordate. All farmed livestock are chordates. We also see a chordate every time we look in the mirror (we are primates, a group of mammals that also includes apes and monkeys). Some extant members of the phylum Chordata—such as lancelets and sea squirts—are, however, much less familiar to most of us. Others are downright repulsive (hagfish and lampreys), though are important for understanding our own evolutionary history. Chordates also include a great variety of bizarre lineages of fish-like forms that went extinct early in the history of the phylum.
This chapter provides a very broad and generalized overview of the diversity and evolutionary history of Chordata, which originated during the Cambrian period alongside most of the other animal phyla. Let's begin here with consideration of basal chordates, which are represented by a great diversity of forms, many of which most people would characterize as fish-like, though none had jaws.
Introduction to Chordate Phylogeny and Synapomorphies
The phylogenetic tree below provides an introduction to the relationships amongst the major groups of chordates. Each of these groups is considered individually below. You may find it useful to refer back to this figure as you learn about each group.
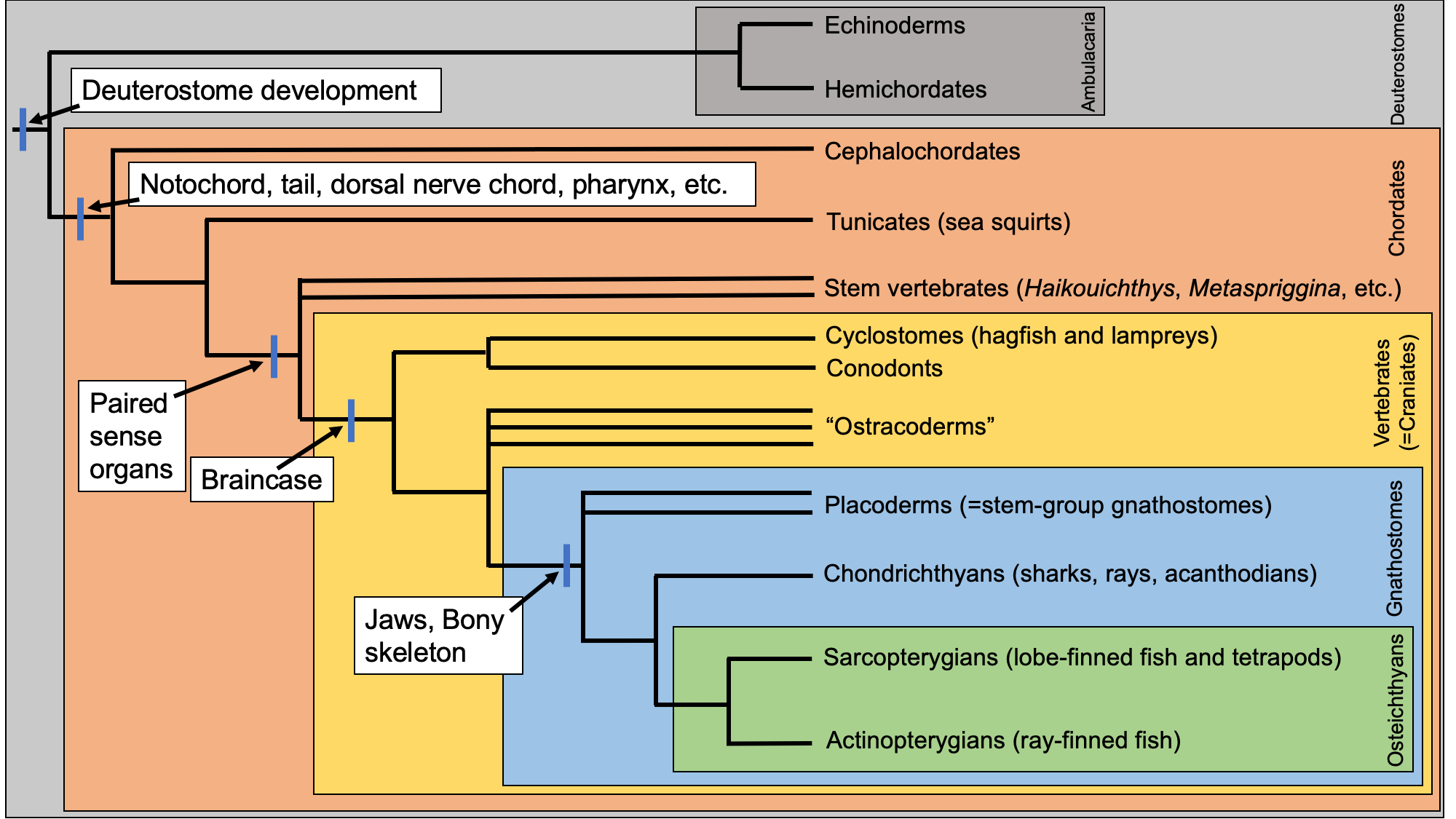
Phylogenetic relationships of major chordate groups. This tree was compiled using several recent publications, including Brazeau and Friedman (2015), Brazeau and de Winter (2015), Janvier (2015), Janvier and Sansom (2016), Donoghue (2017), Miyashita et al. (2019). Image by Jonathan R. Hendricks.
All chordates share a suite a shared, derived features (synapomorphies) in common that all must have also been present in their shared common ancestor. These include:
- Notochord: A flexible supporting rod that spans much of the length of the body and serves as a structure for muscle attachment (among other features); becomes part of the vertebral column in most vertebrates, though is retained through adulthood in some basal taxa.
- Dorsal nerve chord: A nerve chord runs along the dorsal surface of the body (homologous to our backs), rather than the ventral surface (common in some other animal groups).
- Post-anal tail: Chordates have a tail that extends beyond the anal opening.
- Endostyle: A mucus-covered structure in the pharyx that is used for filter feeding in basal chordates and develops into the thyroid gland in derived chordates.
- Pharyngeal slits: Openings into the pharynx (throat region) that are associated with filter feeding in basal chordates and respiration in fishes (in conjunction with gills). Land-dwelling chordates have pharyngeal slits early in embryonic development, but they are subsequently lost. (Note: this feature is also present in hemichordates but is absent in extant members of their sister group, the echinoderms. This means that pharyngeal slits might be a synapomorphy for deuterostomes that was later lost in echinoderms. If true, it means that the character is actually plesiomorphic in chordates. Nevertheless, all chordates have the feature.)
These five features are illustrated in some of the taxa highlighted below.
Cephalochordates
Cephalochordates are represented today by small, sliver-shaped marine animals called amphioxus that park themselves in the sand and filter their food from the water. At first glance, they look a little like worms. A closer examination, however, shows that they have all of the key features that are listed above that are necessary to be classified as chordates. DNA shows us that they are the most basal of all living chordate animals.
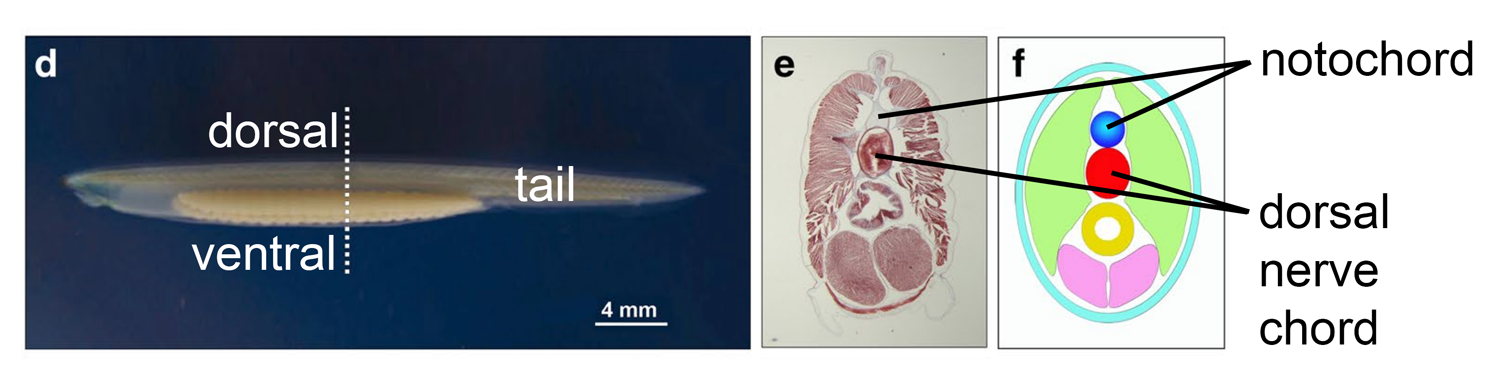
Left: side view of the amphioxus Branchiostoma lanceolatum. Middle and right: internal anatomy of amphioxus shown in cross section (middle, photograph; right, schematic), with the notochord and dorsal nerve chord labeled. Image modified by Jonathan R. Hendricks from original published by Annona et al. (2015, Fig. 1). Original image is associated with a Creative Commons Attribution 4.0 International License.
The video below shows examples of live amphioxus caught near Tampa Bay, Florida, as well as close-up views of their bodies.
"Your Inner Fish | The 500-Million-Year History of the Human Brain" by PBS (YouTube).
Cephalochordates have a really poor, and perhaps nonexistant, fossil record. Modern cephalochordates are completely soft bodied, so this is not terribly surprising. Even so, a small number of taxa from Cambrian lagerstatten have been interpreted to either be cephalochordates, or to have features that suggest cephalochordate affinites. A key problem—as elegantly shown by Sansom et al. (2010)—is that some of the most important features needed to diagnose chordates like modern amphioxus are also among the first to decay after death, hampering our ability to determine whether they are part of the chordate crown group, or rather are stem-group chordates (i.e., basal to Cephalochordata).
Pikaia gracilens, known only from the Middle Cambrian Burgess Shale lagerstatte of British Columbia, is a small (~4 cm) species that is likely important for understanding the features of early chordates, if not cephalochordates.

Lectotype of Pikaia gracilens from the Middle Cambrian Burgess Shale Member of the Stephen Fm., British Columbia (USNM PAL 57628). Note that a single, though separated, specimen is preserved (posterior half [left] rotated relative to anterior half [right]). Image retrieved from iDigBio; copyright Smithsonian Institution.

Reconstruction of Pikaia gracilens by Nobu Tamura (Wikimedia Commons; Creative Commons Attribution-Share Alike 4.0 International license).
Pikaia was made famous by Stephen Jay Gould in his 1989 book Wonderful Life: The Burgess Shale and the Nature of History, where he introduced it in the epilogue as "the world's first known chordate" (p. 322). Wonderful Life is a book about the scientific study of the animals of the Burgess Shale, but more broadly is about the signficance of chance, or contingent, events in the history of life. Gould suggests a thought experiment: "[w]ind the tape of life back to Burgess times, and let it play again. If Pikaia does not survive in the replay, we [as its chordate descendants] are wiped out of future history—all of us, from shark to robin to orangutan" (p. 323).
Subsequent study has complicated the interpretation of Pikaia. It has been interpreted as a cephalochordate, a stem chordate, a stem vertebrate, a vertebrate (see Sansom et al., 2010 and references therein), as well as possibly not part of the chordate clade at all. The key problem is not lack of well preserved fossils (there are many); rather it is the combination of features—some chordate-like, some not—that typify Pikaia.
Pikaia fossils are elongate in shape and show evidence of a notochord, dorsal nerve chord, and v-shaped muscles called myomeres (another common feature of chordates). These characteristics are consistent with cephalochordates. In contrast, two tentacles and nine pairs of small appendages are present at the anterior end of its body, which are not observed in extant amphioxus, leading some paleontologists to question the possible placement of Pikaia among the cephalochordates. Conway Morris and Caron (2012) considered this matter at length, settling on "a scenario that regards Pikaia as the most stem-ward of the chordates" (p. 480), even if not a true cephalochordate. Janvier (2015) characterized Pikaia as "an enigma" and noted that "opinions about its affinities oscillate between the chordate hypothesis and a convergent morphology in some protostomes (the sister group of the deuterostomes)" (p. 484).
Even if Pikaia presents itself as a questionable cephalochordate (or even chordate), another genus—Cathaymyrus—from the Early Cambrian of China provides a somewhat stronger case for being a cephalochordate (or stem-cephalochordate). Even though it is only known from two specimens (assigned to two different species), Cathaymyrus preserves compelling evidence of pharyngeal slits which—as you learned above—are a synapomorphy for chordates.

Reconstruction of Cathaymyrus diadexus by Nobu Tamura (Creative Commons Attribution-Share Alike 4.0 International license).
Tunicates (Sea Squirts)
As adults, most tunicates—also known as sea squirts and ascidians—are sessile animals that attach themselves to the substrate and attain food by filter feeding particles from the water. Some species are solitary, while others are colonial. They don't look anything like other chordates; most look like blobs with two tubes (inhalent and exhalent siphons).
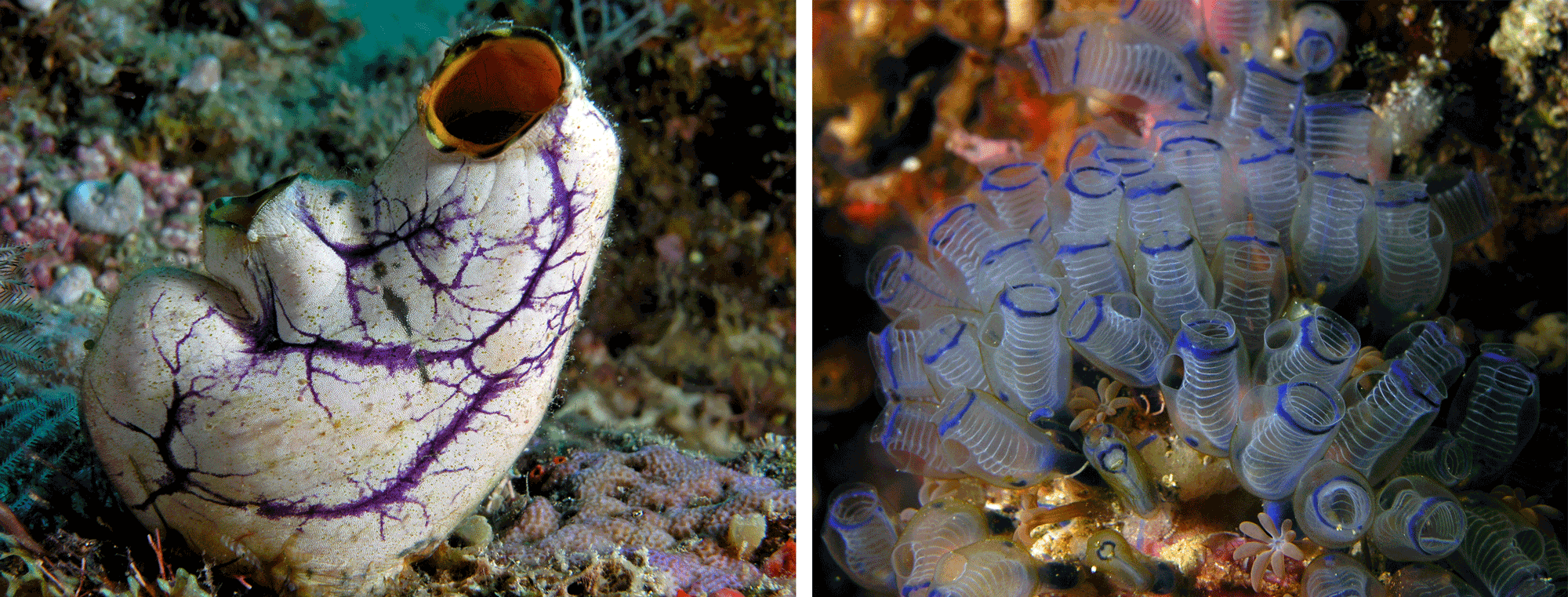
Left: The solitary tunicate Polycarpa aurata; image by Nick Hobgood (WikiMedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license). Right: zooids of the colonial tunicate Clavelina moluccensis; image by Nick Hobgood (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license).
While most sea squirts are small and spend their adult lives stuck to rocks, some species form pelagic colonies—some gigantic—that float through the ocean, filtering food from the surrounding water.
"Mysterious Sea 'Worm' Spotted Near New Zealand" by National Geographic (YouTube).
So, why are tunicates classified as chordates? First, molecular phylogenetic analyses consistently place them at a basal position within the Chordata. Biologists, however, long predicted this relationship based on morphological analyses of the mobile, tadpole-like larvae of tunicates, which have most of the synapomorphies of chordates that are listed above, including a tail, notochord, dorsal nerve chord, endostyle, and a pharyx. The endostyle is retained in the adult form, as is the pharynx, which bears gill slits.
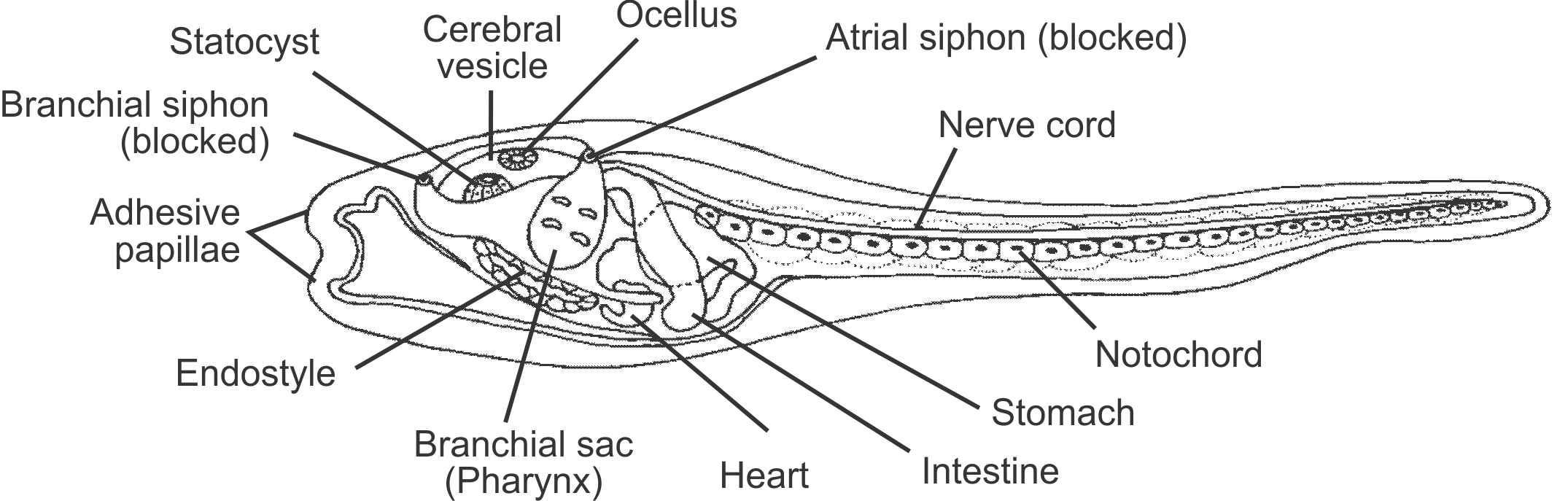
Morphology of a tunicate larva, with important features labeled. Image by Jon Houseman (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license).
Tunicates have a very poor fossil record, though a handful of specimens have been attributed to the group. One of these is Shankouclava from the Early Cambrian of Kunming, South China (Chen et al., 2003).
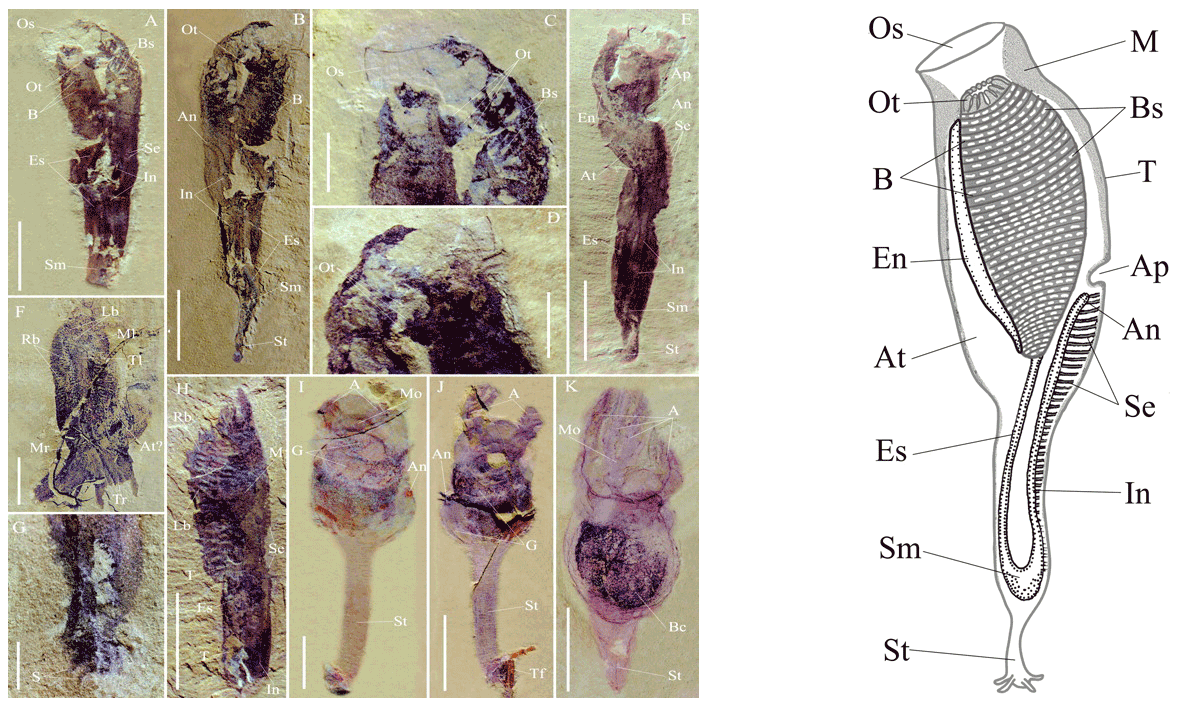
Left: Fossil specimens of the purported Early Cambrian tunicate Shankouclava from Kunming, China. Right: Reconstruction of Shankouclava. Important labeled features include: Bs, branchial slits; En, endostyle; and Ph, pharynx. Both images are from Chen et al. (2003; figs. 1 and 3) in PNAS, which allows for re-use of images for educational purposes (see here).
Stem Vertebrates
A handful of important, fish-like fossils from Cambrian lagerstatten deposits have shed light on the early history of vertebrate evolution. These include fossils such as Myllokunmingia, Haikouichthys, and Haikouella from the Early Cambrian Chengjiang Lagerstatten of South China and Metaspriggina from the Middle Cambrian Burgess Shale of British Columbia, Canada. Most phylogenetic analyses (e.g., Conway Morris and Caron, 2014) have placed these taxa in a position that is immediately basal to crown group vertebrates. Therefore, these animals are regarded as stem vertebrates, rather than true vertebrates. While they have some important features of true vertebrates (e.g., a head with paired sensory organs such as eyes), they lack evidence of having a skull, which is a synapomorphy of crown group vertebrates (hagfish and lampreys, which are the most basal living vertebrates, have cartilaginous skulls; see next page).
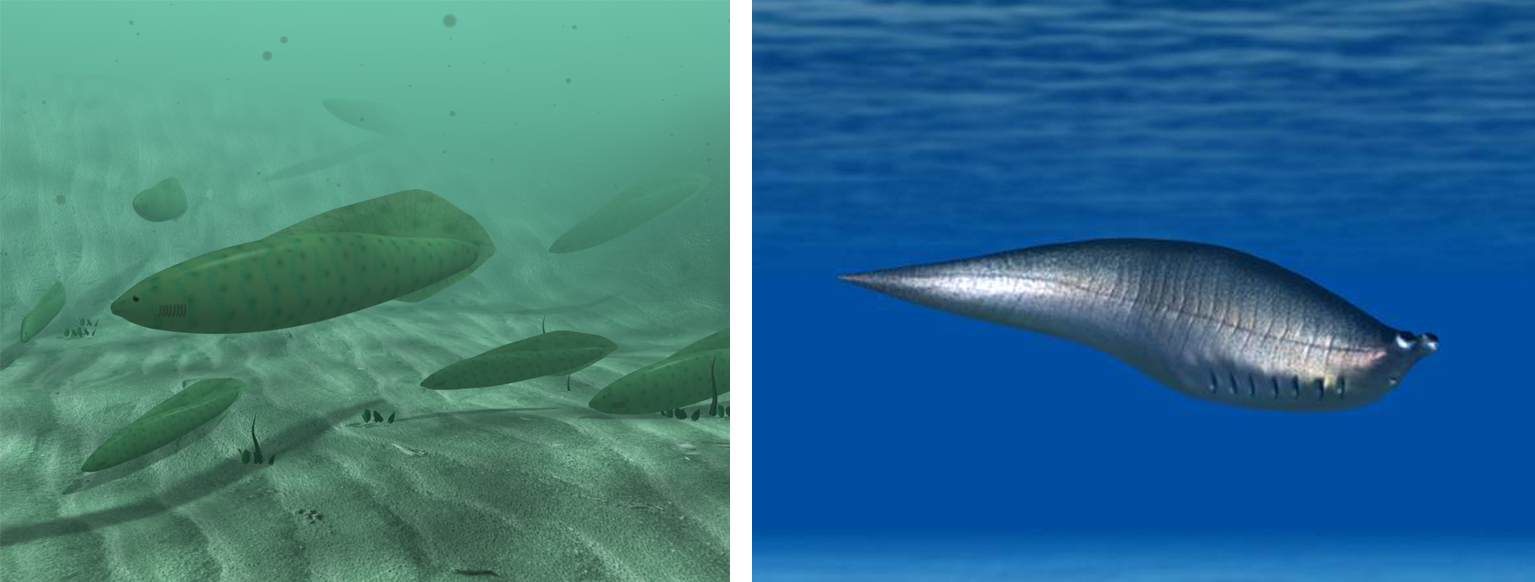
Cambrian stem vertebrates. Left: reconstruction of Haikouichthys ercaicunensis from the Early Cambrian of China; image by "Talifero" (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license). Right: reconstruction of Metaspriggina walcotti from the Middle Cambrian of British Columbia, Canada; image by Nobu Tamura (Wikimedia Commons; Creative Commons Attribution-Share Alike 4.0 International license).
References and Further Reading
Annona, G., N.D. Holland, and S. D'Aniello. 2015. Evolution of the notochord. EvoDevo 6:30.
Brazeau, M. D., and V. de Winter. 2015. The hyoid arch and braincase anatomy of Acanthodes support chondrichthyan affinity of 'acanthodians'. Proceedings of the Royal Society B 282: 20152210.
Brazeau, M. D., and M. Friedman. 2015. The origin and early phylogenetic history of jawed vertebrates. Nature 520: 490-497.
Chang, M., F. Wu, D. Miao, and J. Zhang. 2014. Discovery of fossil lamprey larva from the Lower Cretaceous reveals it three-phased life cycle. Proceedings of the National Academy of Sciences of the United States of America 111(43): 15486-15490.
Chen, J.-Y., D.-Y. Huang, Q.-Q. Peng, H.-M. Chi, X.-Q. Wang, and M. Feng. 2003. The first tunicate from the Early Cambrian of South China. Proceedings of the National Academy of Sciences of the United States of America 100(14): 8314-8318.
Conway Morris, S., and J.-B. Caron. 2012. Pikaia gracilens Walcott, a stem-group chordate from the Middle Cambrian of British Columbia. Biological Reviews 87: 480-512.
Conway Morris, S., and J.-B. Caron. 2014. A primitive fish from the Cambrian of North America. Nature 512: 419-422. [Note: this paper redescribes Metaspriggina.]
Donoghue, P. C. J. 2017. Evolution: Divining the nature of the ancestral vertebrate. Current Biology 27: R259-281.
Donoghue, P. C. J., and J. N. Keating. 2014. Early vertebrate evolution. Palaeontology 57(5): 879-893.
Gess, R.W., M.I. Coates, and B.S. Rubidge. 2006. A lamprey from the Devonian period of South Africa. Nature 443: 981-984.
Janvier, P. 2015. Facts and fancies about early fossil chordates and vertebrates. Nature 520: 483-489.
Janvier, P., and R. S. Sansom. 2016. Fossil hagfish, fossil cyclostomes, and the lost world of "ostracoderms". Pp. 73-93 in: Edwards, S. L., and G. G. Goss, Hagfish Biology, CRC Press.
Miyashita, T., M. I. Coates, R. Farrar, P. Larson, P. L. Manning, R. A. Wogelius, N. P. Edwards, J. Anné, U. Bergmann, A. R. Palmer, and P. J. Currie. 2019. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proceedings of the National Academy of Sciences of the United States of America 116(6): 2146-2151.
Sansom, R.S., S.E. Gabbott, and M.A. Purnell. 2010. Non-random decay of chordate characters causes bias in fossil interpretation. Nature 463: 797-800.
Shu, D.-G., Conway Morris, S., and X.-L. Zhang. 1996. A Pikaia-like chordate from the Lower Cambrian of China. Nature 384: 157-158.
Acknowledgments
Thank you to Philip Donoghue for comments that improved this chapter and Matthew Friedman for providing helpful notes that facilitated development of the page about jawless vertebrates. Any mistakes in this chapter are, however, entirely the fault of the author.
Content usage
Usage of text and images created for DEAL: Text on this page was written by Jonathan R. Hendricks. Original written content created by Jonathan R. Hendricks for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images created by Jonathan R. Hendricks are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.



