Section contents:
- Origin of land plants ←
- The land plant life cycle
- Greek and Latin in botanical terminology
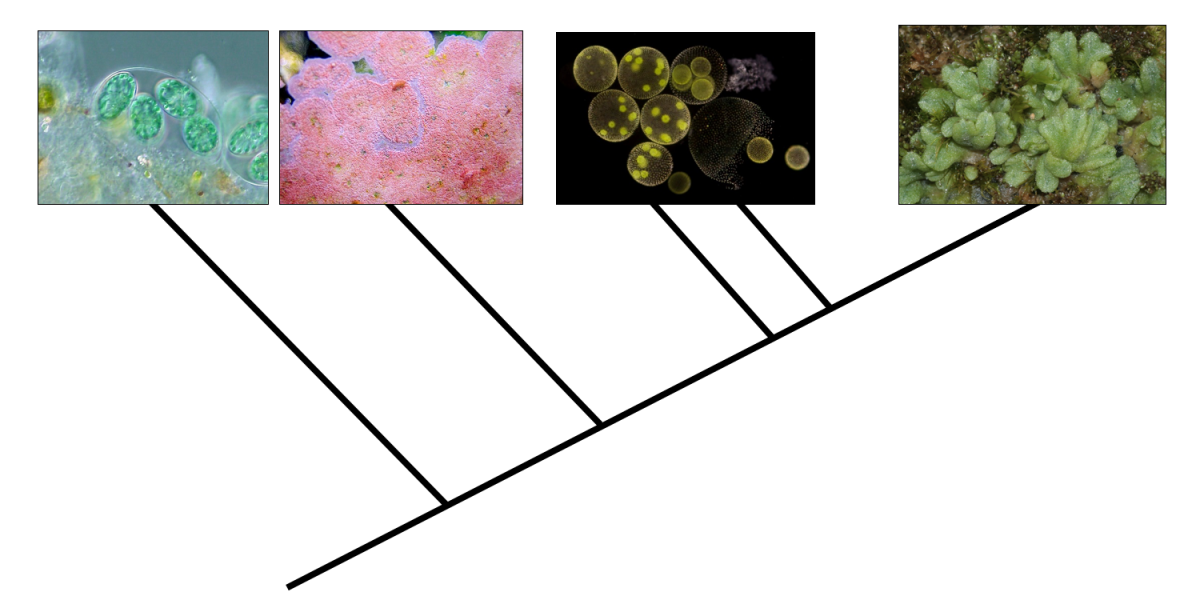
Feature image: Tree of relationships in Kingdom Plantae. From left to right: glaucophyte algae, red algae, green algae, and land plants. Credits: Glaucocystis (NEON, via Wikimedia Commons, CC BY-SA 2.5); coralline algae (FalsePerc, via Wikimedia Commons, CC BY-SA 3.0); Volvox (Frank Fox, via Wikimedia Commons, CC BY-SA 3.0 DE); Riccia sorocarpa, a liverwort (HermannSchachner, Wikimedia Commons, CC0 1.0).
What is a plant?
Defining which organisms qualify as plants is not necessarily straightforward. For example, we could attempt to define a plant as an organism that generates its own food through the process of photosynthesis. Many types of organisms, however, are photosynthetic. These include cyanobacteria ("blue-green algae"), single-celled eukaryotic organisms like dinoflagellates and Euglena, and complex multicellular seaweeds like kelp. Many of these organisms are not closely related to one another, nor to plants that live on land (like ferns, conifers, and flowering plants). So what makes a plant a plant?
Colloquially speaking, the term "plant" often refers to organisms that are here called "land plants," or embryophytes. Embryophytes are multicellular, autotrophic (with few exceptions), typically terrestrial organisms that retain and nourish their embryos (hence the name). The land plants are part of a broader monophyletic group that includes several types of algae. Algae are photosynthetic organisms that are not land plants (yes, this explanation is a bit circular). There are many types of algae, and they occur in several unrelated groups. The algae that are in a monophyletic group with land plants are the glaucophytes, an obscure group of unicellular algae; the red algae, mostly marine species that commonly appear red due to the presence of the pigment phycoerythrin; and the green algae, a paraphyletic group from which the land plants originated. Together, the land plants and these related algae are considered part of Kingdom Plantae, or the group Archaeplastida ("ancient plastid").
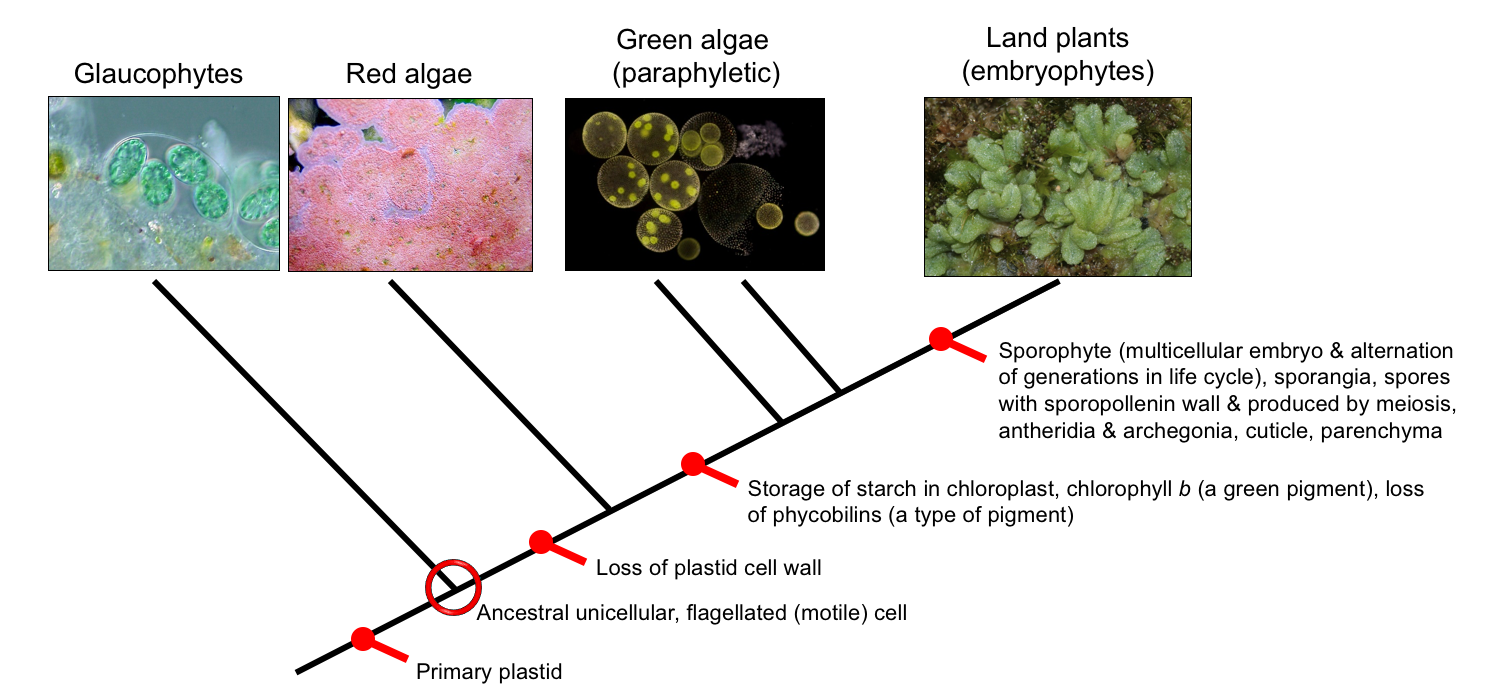
Kingdom Plantae (a.k.a. the Archaeplastida). Tree of relationships, showing the four major groups of Kingdom Plantae. From the base to the top of the tree, these are the glaucophytes, red algae, green algae (a paraphyletic group that excludes the land plants), and land plants or embryophytes. This entire clade is defined by the synapomorphy of having a primary plastid (chloropast). Credits: Glaucocystis (NEON, via Wikimedia Commons, CC BY-SA 2.5); coralline algae (FalsePerc, via Wikimedia Commons, CC BY-SA 3.0); Volvox (Frank Fox, via Wikimedia Commons, CC BY-SA 3.0 DE); Riccia sorocarpa, a liverwort (HermannSchachner, via Wikimedia Commons, CC0 1.0). Images modified from originals.
All of the groups in Kingdom Plantae share the characteristic of having a primary chloroplast or primary plastid. Plastids are a type of membrane-bound structure (organelle) inside a plant or algal cell. They contain genetic material (DNA) and self-replicate by dividing in a process known as binary fission. Plastids can play a variety of roles in a cell. Chloroplasts (from the Greek chloros = green) contain pigments that function in the process of photosynthesis. Other types of plastids are developmentally related to or derived from chloroplasts, and may contain other pigments, make starch, etc.
Chloroplasts are descended from a type of single-celled organism called a cyanobacterium. Cyanobacteria are photosynthetic bacteria that are sometimes called "blue-green algae" (cyano-, from the Greek kyanos, indicates a blue color). Ancient cyanobacteria added oxygen, a byproduct of photosynthesis, to the atmosphere early in Earth's history. Cyanobacteria are still biologically and ecologically significant today. Certain of these bacteria, particularly Microcystis, can produce toxic blooms when they proliferate in freshwater. Some cyanobacteria are capable of nitrogen fixation, a process that converts atmospheric nitrogen into a form that can be used by land plants (read more here).
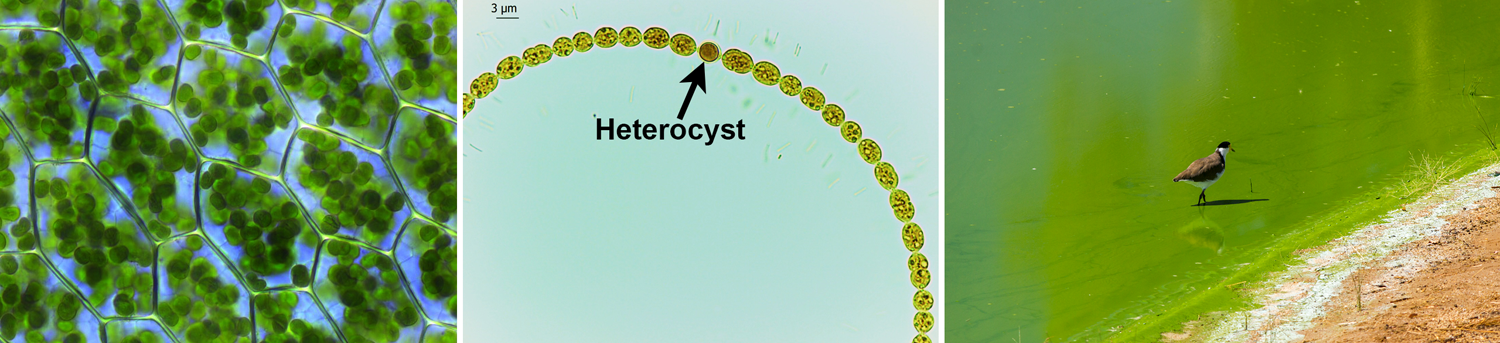
Chloroplasts and cyanobacteria. Left: Chloroplasts (circular, green bodies) in the cells of a thyme-moss (Plagiomnium affine). Center: A filamentous cyanobacterium (Anabaena) with a specialized cell called a heterocyst; the heterocyst is where nitrogen fixation takes place. Right: A green bloom formed by a toxin-producing cyanobacterium (Microcystis aeruginosa) in a lake. Credits: Plagiomnium affine leaf cells (Kristian Peters--Fabelfroh, via Wikimedia Commons, CC BY-SA 3.0); Anabaena-Necklace of Mermaid (Veryn4ik89, via Wikimedia Commons, CC BY-SA 4.0); Vanellus miles standing in Microcystis aeruginosa outbreak (Bidgee, via Wikimedia Commons, CC BY-SA 3.0 AU). Images modified from originals.
The theory that explains how the plant cell obtained its chloroplast is known as endosymbiotic theory (from the Greek endon = within + syn = together + biōsis = living). The chloroplast is thought to have originated when an ancient cyanobacterium was engulfed by another cell. Instead of being digested, the cyanobacterium lived inside the engulfing cell in an endosymbiotic relationship, which may have been mutually beneficial. For example, the host cell may have received food or oxygen from the cyanobacterium, whereas the cyanobacterium may have benefitted from a protected environment or carbon dioxide provided by the host.
Through many generations, the descendants of the cyanobacterium and its host became more integrated, eventually losing the ability to live separately. We know that modern chloroplasts descended from cyanobacteria in part because they retain some evidence of their origins as independent organisms. Some types of evidence that the chloroplast (or plastid) is descended from a free-living cyanobacterium are:
- Chloroplasts/plastids have their own bacterium-like (circular) chromosome similar to that found in cyanobacteria.
- Chloroplasts/plastids duplicate themselves by division in a manner similar to free-living bacteria (binary fission).
- Primary chloroplasts/plastids are surrounded by two layers of membranes that are equivalent to the membranes surrounding a cyanobacterial cell.
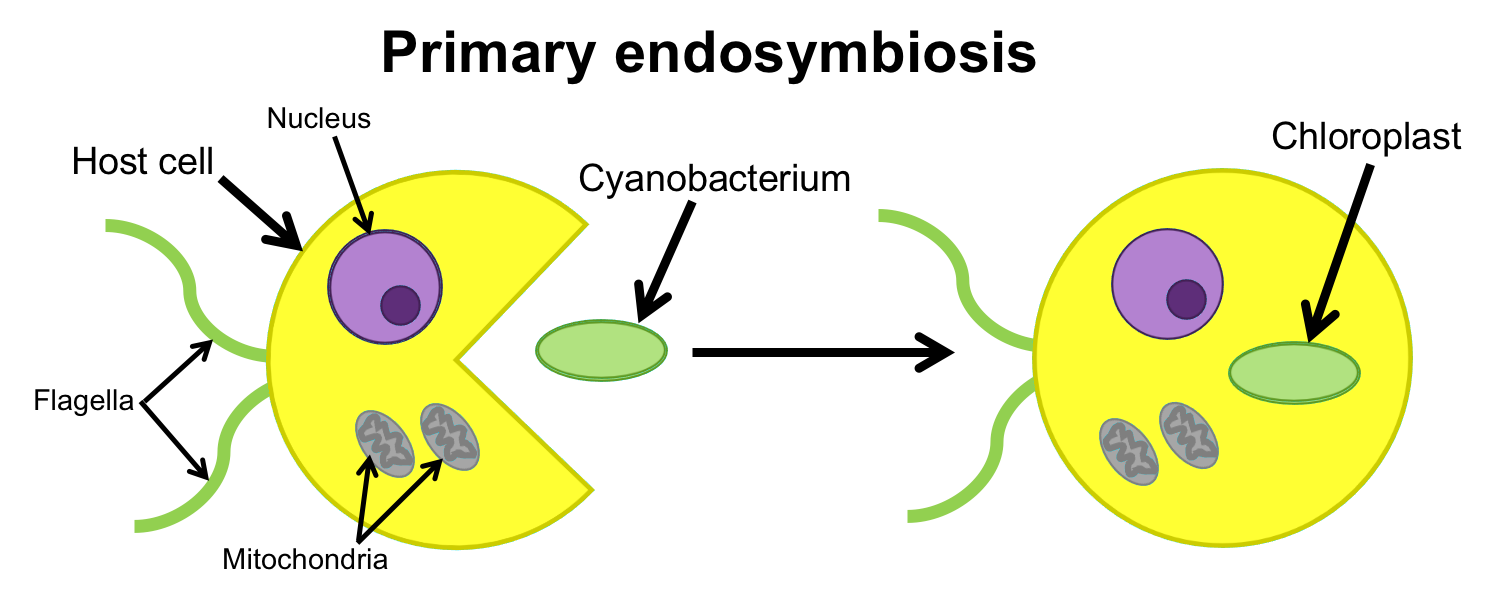
Endosymbiosis & the origin of the chloroplast. The primary chloroplast found in plant cells originated when an ancient unicellular organism engulfed a cyanobacterium, which lived inside the host. Over generations, the descendants of the host and the endosymbiotic cyanobacterium became more integrated, until finally they were incapable of living apart. Key to other labelled structures: Flagella are whip-like cellular structures used in cellular locomotion. The nucleus is the so-called "control center" of the cell that contains chromosomes. The mitchondrion is another type of organelle that, like the plastid, has its own DNA and self-replicates. It was acquired through a more ancient endosymbiotic event than the plastid and is found in the cells of many types of organisms, including animals, plants, fungi, etc. Credit: Diagram by E.J. Hermsen (DEAL).
Video explaining the process of endosymbiosis in the origin of the eukaryotic cell. This video describes how the complex cells of eukaryotes (plants, animals, fungi, and many other types of organisms) evolved through the process of endosymbiosis. Endosymbiosis explains both the origin of the mitochondrion and the origin of the chloroplast. Credit: How Two Microbes Changed History (PBS Eons, via YouTube).
The plant chloroplast is considered primary because it descends from this original endosymbiotic relationship between host cell and cyanobacterium. It is thought that the ancestors of today's plant chloroplasts may have become endosymbionts over one billion years ago (see here). Most other photosynthetic algae outside of Kingdom Plantae (for example, diatoms, kelps, and dinoflagellates) have secondary chloroplasts that were gained when a host cell engulfed another cell that already contained a primary chloroplast (usually, a one-celled type of red or green alga). In a few cases, algae have tertiary chloroplasts, meaning that a cell engulfed a cell whose ancestor had engulfed a cell whose ancestor had engulfed a cyanobacterium! (Did you get all of that? Read more here.)
Beyond the chloroplast and the ability to generate sugars through the process of photosynthesis, members of Kingdom Plantae do not have many distinctive unifying features. They exhibit a great variety of growth forms and life cycle types. Land plants, or embryophytes, are separated from closely related algae by their life cycle and structural features, many of which are related to their transition to and subsequent diversification on land.
Origin of the land plant sporophyte
The land plant life cycle is haplodiplontic (Greek, haploos + diploos + einai = single double being) and shows alternation of generations. This means that in order to complete a full circle of the life cycle, a land plant has to pass through two distinct, multicellular adult generations: a diploid sporophyte generation (spore-producing plant) and a haploid gametophyte generation (gamete-producing plant). A diploid organism has two sets of chromosomes in its cells, while a haploid organism has one set of chromosomes in its cells.
The steps in the land plant life cycle are as follows:
- The diploid sporophyte produces haploid spores by meiosis. Meiosis is a special type of cell division that halves the number of sets of chromosomes per cell. In meiosis, one diploid parent cell divides to yield four haploid daughter cells. The daughter cells are genetically different from each other.
- The spores germinate and grow into multicellular haploid gametophytes.
- The gametophytes produce haploid gametes (sex cells). In land plants, the gametes are larger, nonmotile eggs and smaller sperm. In many land plants, including the earliest plants, the sperm are motile (in other words, they swim).
- A sperm cell fertilizes an egg cell, yielding a diploid cell called a zygote.
- The zygote begins to divide and grow, eventually developing into the next generation of sporophyte.
The land plant life cycle is said to have "alternation of generations," because the sporophyte and gametophyte generations alternate with one another in order to fully complete the process of sexual reproduction. The land plant life cycle is explained in more detail here.
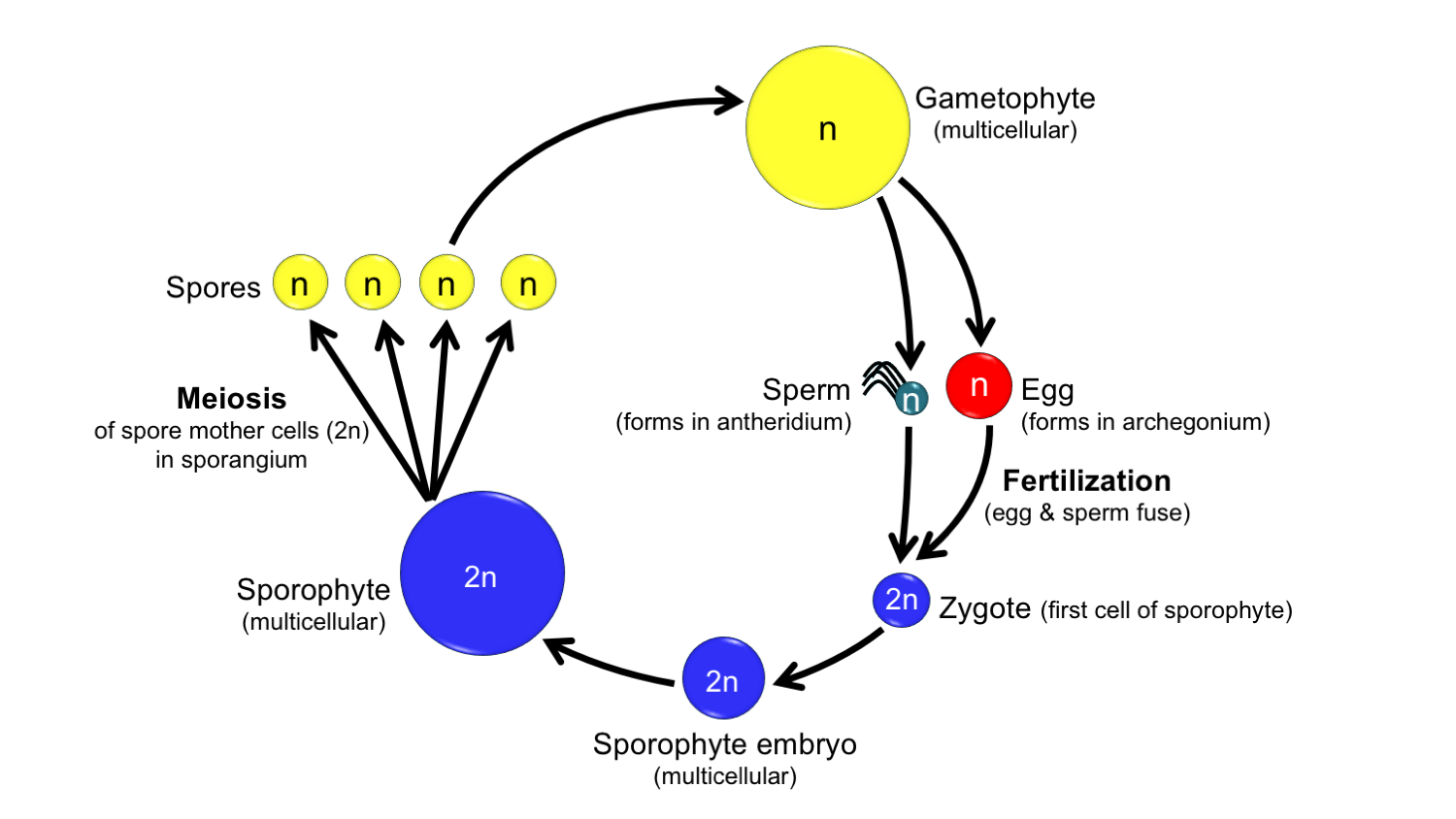
Generalized land plant life cycle. In order to complete a full turn of its life cycle, a land plant must pass through a diploid sporophyte (spore-producing) generation, and a haploid gametophyte (gamete-producing) generation. Diploid = each cell has two sets of chromosomes = 2n; haploid = each cell has one set of chromosomes = n. Credit: Diagram by E.J. Hermsen (DEAL).
Algal ancestors of land plants
The land plants arose from the green algae, and, together, land plants and green algae are sometimes called "Viridiplantae" (from the Latin viridis = green). The main candidates for the living green algal group most closely related to land plants are the stoneworts (order Charales), order Coleochaetales, and the conjugating green algae (order Zygnematales). Charales and Coleochaete in particular show some compelling structural similarities to land plants (for example, connections called plasmodesmata between cells); their gametes are also differentiated into smaller, motile sperm and larger, nonmotile eggs (a condition known as oogamy), as in many land plants. The close relationship between these green algal groups and land plants is supported by molecular phylogenetic analyses.

Green algae closely related to land plants. Left to right: Chara, a branching filamentous form (Charales); drawing of Coleochaete (Coleochaetales), a flat, disk-like form; Spirogyra (Zygnematales), an unbranched filamentous form; and Micrasterias (Zygnematales), a unicellular form. Credits: Chara braunii (Show_ryu, via Wikimedia Commons, CC BY-SA 3.0); Coleochaete orbicularis from Cooke (1882-1884) British fresh-water algae, exclusive of Desmidieae and Diatomaceae, vol. 2 (via Wikimedia Commons, no known copyright restrictions); Spirogyra (Wiedehopf20, via Wikimedia Commons, CC BY-SA 4.0); Micrasterias (Frank Fox, via Wikimedia Commons, CC BY-SA 3.0 DE). Images modified from originals.
These algae offer clues to the characteristics that might have occurred in the algal ancestor of the land plants. Most are freshwater forms, and some are multicellular. They are all haploid organisms that have a haplontic (Greek, haploos + einai = single being) life cycle. Thus, the ancestor of land plants is thought to have been a multicellular, freshwater, haploid alga sharing some features with land plants (oogamy, plasmodesmata, etc.). However, it likely differed from land plants in having a haplontic life cycle.
A haplontic life cycle can be summarized as follows:
- Haploid organisms produce haploid gametes, or sex cells.
- The gametes fuse in the process of fertilization to form a diploid cell called a zygote.
- The zygote undergoes meiosis to produce haploid daughter cells.
- The haploid daughter cells grow into the next generation of haploid organisms.
Because the green algae most closely related to land plants have only a haploid, gamete-producing generation, their life cycle includes only the equivalent of the gametophyte generation in land plants. It is thus reasonable to conclude that the sporophyte is a novel innovation in the land plant lineage. So where did the sporophyte come from?
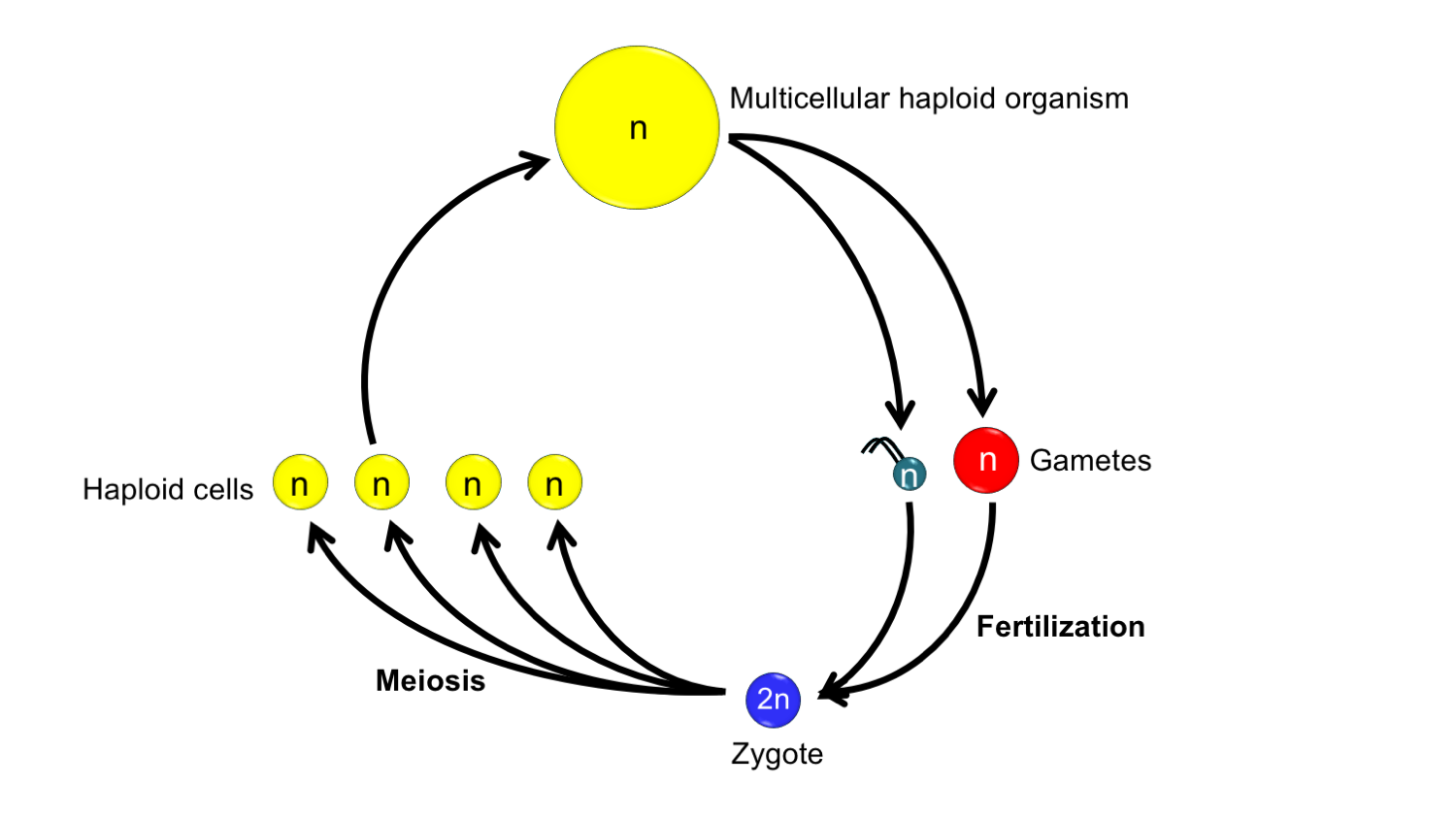
Hypothesized haplontic life cycle in the ancestor to land plants. In this life cycle, a multicellular haploid organism gives rise to gametes (sex cells, in this organism eggs and sperm). The gametes fuse to form a zygote. The zygote undergoes meiosis to produce haploid cells. The haploid cells grow into haploid multicellular organisms to complete the life cycle. Credit: Diagram by E.J. Hermsen (DEAL).
The interpolation theory of sporophyte evolution
One theory that explains the origin of the sporophyte in land plants is known as the interpolation theory or the antithetic theory. In this theory, the origination of the sporophyte began when the zygote in the life cycle of a multicellular, haploid green alga divided by mitosis instead of meiosis. Mitosis is simple cell division in which one parent cell divides to produce two identical daughter cells. Thus, mitotic divisions of the diploid zygote would have produced a group of identical diploid cells. At least some of these cells would have then undergone meiosis to produce haploid cells and complete the life cycle.
This rudimentary cluster of diploid cells was the first phase in the evolution of the sporophyte. Eventually, the sporophyte become permanently inserted, or interpolated, as an extra step in the land plant life cycle and began to increase in complexity.
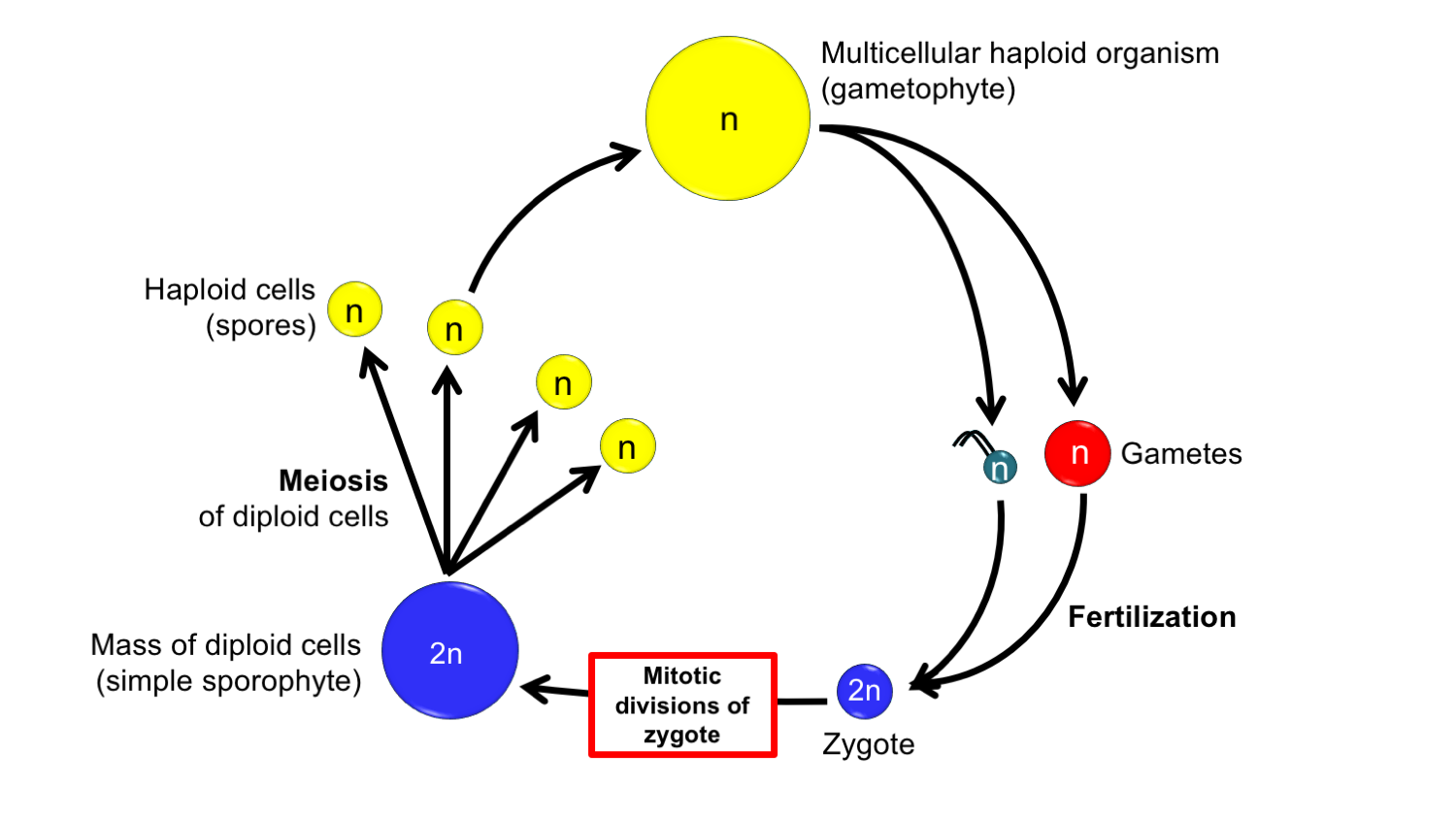
Interpolation theory. The interpolation theory posits that the sporophyte in land plants was inserted into the haplontic life cycle of a green alga. The sporophyte first arose when the zygote did not immediately undergo meiosis, but instead underwent a period of replication through mitosis (basically, cell division in which the zygote produced copies of itself) before meiosis occurred. In its earliest and simplest form, all of the cells of the sporophyte may have undergone meoisis to produce spores. Credit: Diagram by E.J. Hermsen (DEAL).
Under the interpolation theory, one might make a few predictions about the earliest sporophytes. First of all, the earliest sporophyte and gametophyte generations would have been heteromorphic generations (from the Greek heteros + morphē = different + form). In other words, they would likely have looked different from one another, since the sporophyte was a new step inserted in the life cycle. Secondly, that the earliest sporophytes might have been relatively short-lived and simple in structure, whereas the corresponding gametophytes would have been more complex and persistent organisms.
The predictions of the interpolation theory are consistent with the interpretation that the earliest land plants had bryophyte-like life cycles. Bryophytes (Greek, bryon + phyton = moss plant) are the three basally diverging groups of living land plants: hornworts, liverworts, and mosses. In bryophytes, the gametophyte is green and persistent; in other words, the gametophyte is the generation that you would recognize as a plant. The sporophyte develops on and is dependent on the gametophyte. It is very simple, has one spore capsule, and exists only to produce spores. The two generations (sporophyte and gametophtye) look very different from one another.

Gametophyte and sporophyte of a liverwort (Marchantia). Left: The gametophyte, which grows as a flat thallus. The cup-like structures contain gemmae, which are structures involved in asexual reproduction. Right: Longitudinal section of sporophytes attached to a Marchantia gametophyte. Note that each sporophyte has a short stalk and a single sporangium (spore-producing capsule). Credits: Umbrella liverwort-Marchantia polymorpha (Pseudopanax, via Wikimedia Commons, Public Domain); Marchantia sporophytes (Jon Houseman & Matthew Ford, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Fossil evidence for the first sporophytes
Dispersed land plant spores, which are surrounded by a resistant sporopollenin wall, are important for tracing the early evolution of land plants in the fossil record. Sporopollenin is also, however, found in some green algal groups. Thus, land plant spores must be distinguished from algal structures in the fossil record in order to identify the earliest land plants.
One unmistakable indication that a spore belongs to a land plant is the presence of a trilete mark. Because land plant spores are the products of meiosis, they are produced in tetrads, or groups of four. The four spores are often packed into a tetrahedral arrangement, like a small pyramid. The Y-shaped trilete mark forms at the contact point among the adjoining spores, which typically separate from one another prior to dispersal. Trilete marks are found on the spores of many living and fossil land plants.
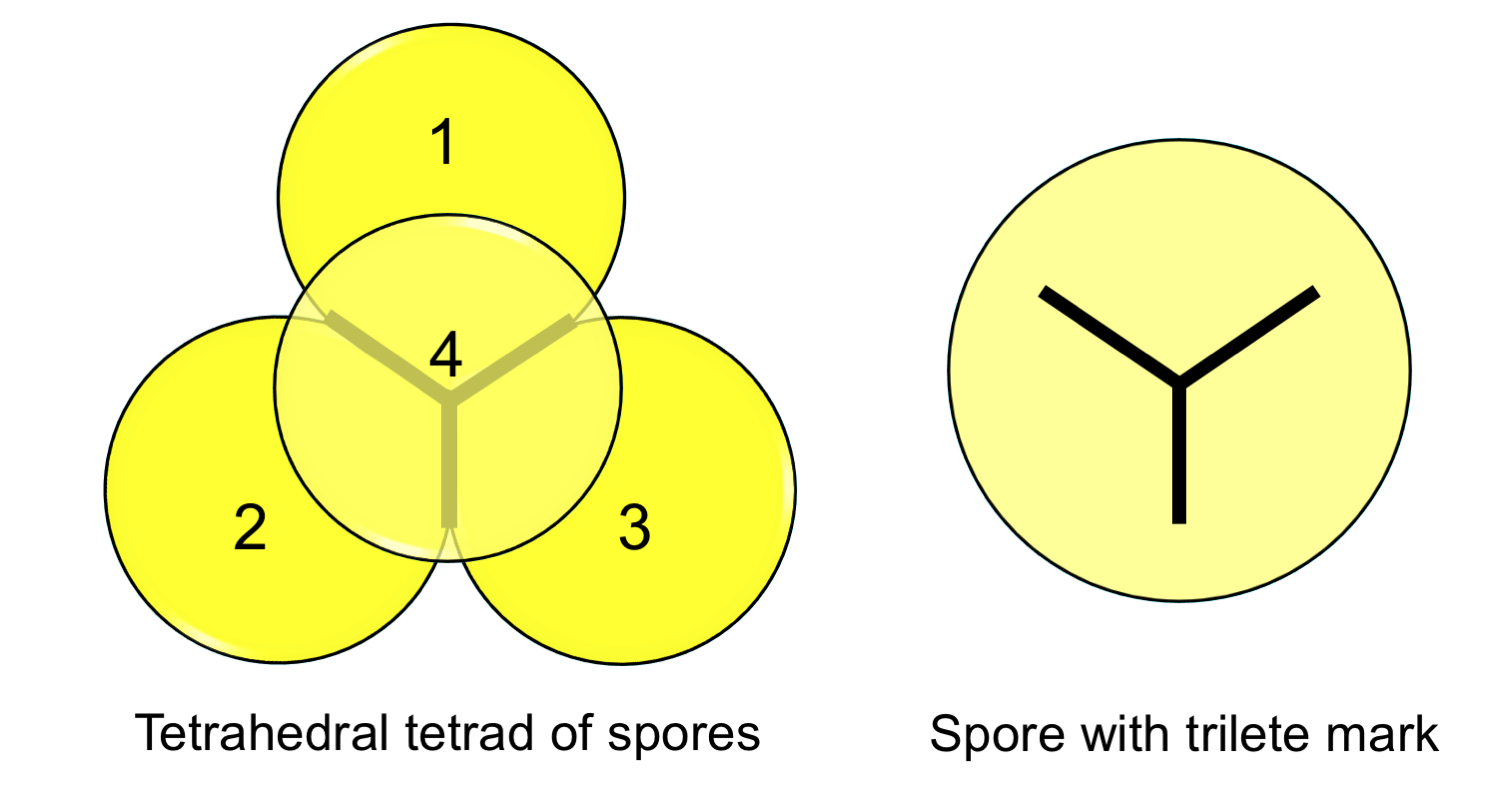
Spore tetrad and trilete mark. Left: A tetrahedral tetrad (group of four) spores. The uppermost spore is semi-transparent to show the Y-shaped contact between the spores, which is emphasized with a thick line. Right: Single spore with trilete mark. Credit: E.J. Hermsen (DEAL).
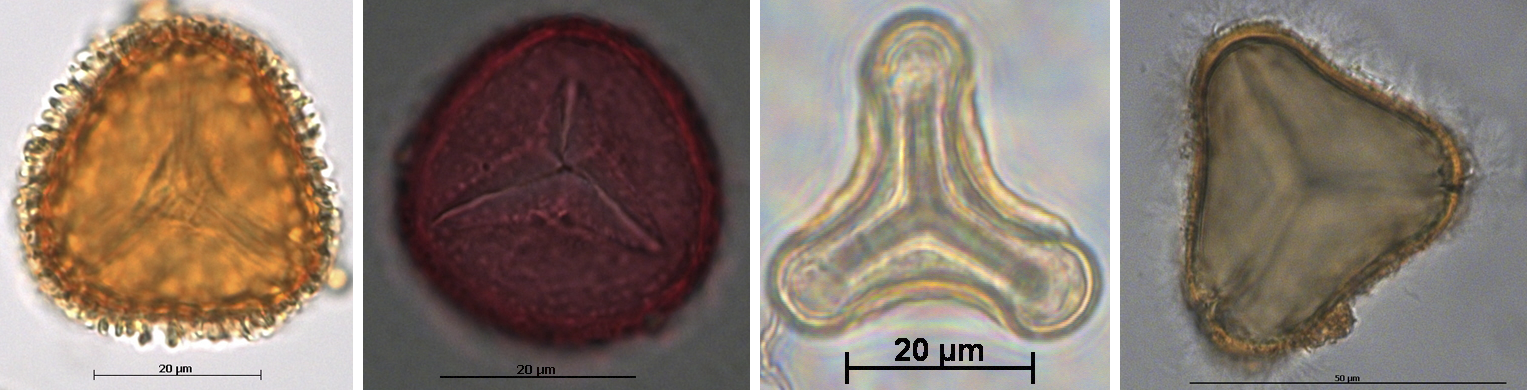
Trilete spores of living land plants. Examples of spores from modern free-sporing plants (plants that release their spores), showing trilete marks. From left to right: Alpine club moss (Lycopodium fastigiatum, APSA sample 401-1-27), limestone adder's-tongue (Ophioglossum engelmanii, APSA sample 404-1-1-7), Old World forked fern (Dicranopteris linearis, APSA sample 407-17-1-6), and rough tree fern (Cyathea australis, APSA sample 407-18-1-1). Credits: All images from the Australasian Pollen and Spore Atlas (CC BY-NC-SA 3.0). Images modified from originals.
The oldest land plant spores, however, lack a trilete mark and are called cryptospores (crypto- from the Greek kryptós, hidden). Cryptospores may have been produced by several different types of organisms. Particularly in the Cambrian, it is unlikely that they are land plant spores, although some might have been produced by green algae closely related to land plants (see here). At least some cryptospores are thought to have been produced by early land plants because they are found in terrestrial deposits, and they share features with spores produced by living bryophytes.
The earliest generally accepted land plant cryptospores are dispersed spores that are over 470 million years old (Middle Ordovician) and are thought to have been made by liverwort-like plants (see here). Beginning in the Late Ordovician, some types of cryptospores have been discovered within possible land plant sporangia (see here), although the identity of these "sporangia" is somewhat debatable (see here). Trilete spores also appear in the Late Ordovician. By comparison, dates derived from phylogenetic analysis of living plants suggest an origin for land plants in the Cambrian to Early Ordovician (see here).

Ordovician cryptospores. Left: Isolated cryptospore (Gneudnaspora divellomedia). Center: Cryptospores in a dyad, or group of two (Segestrespora laevigata). Right: Cryptospores in a tetrad, or group of four (Tetrahedraletes medinensis). Credits: Gneudnaspora divellomedia, Segestrespora laevigata, and Tetrahedraletes medinensis by PhilSteem (via Wikimedia Commons, CC BY-SA 4.0). Images modified from originals.
No fossil evidence preserving the structure of the earliest, simplest sporophytes has been discovered. There is a big time gap between when the first land plant cryptospores are recorded and the first sporophyte body fossils appear. Most of the fossil evidence documenting the structure of early land plant sporophytes comes from the Silurian to Devonian periods. Thus, the structural evolution of the earliest sporophytes is somewhat speculative (see here for a review).
As discussed above, the earliest stage in sporophyte evolution may have been a simple cluster of diploid cells. Each of these cells may have undergone meiosis to produce spores. Over time, the sporophyte cells may have become more specialized, so that some cells were reproductive (underwent meiosis to produce spores), whereas other cells were vegetative (non-reproductive, or non-spore producing).
As they increased in complexity, ancient land plant sporophytes may have begun to resemble the sporophytes of bryophytes, particularly those of some liverworts and mosses. Thus, a sporophyte would have been a simple organism with a single, unbranched stalk bearing one sporangium (spore-producing capsule) at its tip. It may have been anchored in the gametophyte tissue by an enlarged foot at the base of the stalk, as in modern bryophytes. Early sporophytes were probably dependent on their mother gametophytes for nutrition. In other words, they would not have been able to sustain themselves independently through photosynthesis, but would have absorbed nutrients from the gametophyte.
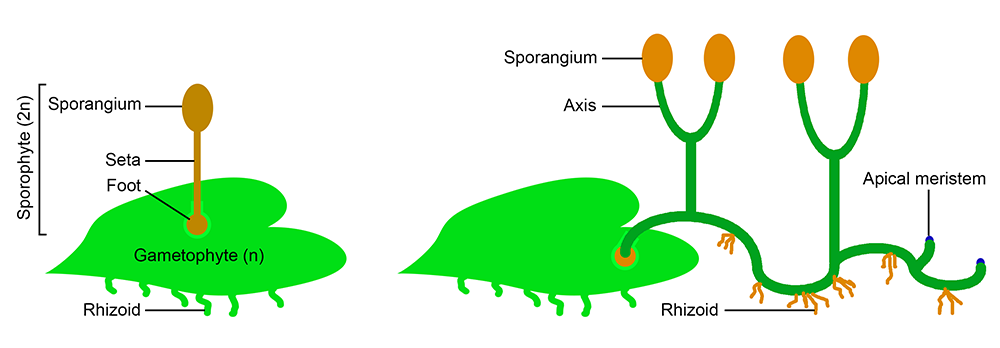
Early evolution of the sporophyte. Left: A typical bryophyte-like sporophyte, probably similar to some of the earliest land plant sporophytes. The sporophyte consists of a foot, which anchors the sporophyte in the gametophyte tissue and absorbs nutrition; a seta, or stalk; and a sporangium, or spore capsule. Some bryophyte sporophytes lack even a stalk, and consist of a foot and sporangium. Right: Sporophyte of an early polysporangiophyte (discussed below). The sporophyte would have begun its life attached to and drawing nutrition from the gametophyte. However, it would eventually have become independent. Note that the sporophyte has apical meristems, or regions where apical growth occurs. Also note that the sporophyte is green (photosynthetic) and anchored to the ground by rhizoids. Rhizoids are anchoring structures consisting of one cell or a row of cells (contrast to roots, which are more complex structures found in vascular plants). Credit: Diagram by E.J. Hermsen (DEAL), based on Figure 9 in Ligrone et al. (2012).
Other sporophyte innovations
Stomata, or pores that are surrounded by guard cells, were an early sporophyte innovation for life on land. Stomata allow for the exchange of gases. In many plants, the guard cells control the opening and closing of the stomata to balance the gas exchange necessary to carry on life processes (i.e., photosynthesis) against the loss of water. Stomata are found in all living plant sporophytes except the sporophytes of liverworts, which are thought to be the first-diverging group of living land plants. It is thought that stomata may have appeared in the Ordovician, and they can be observed on some Silurian sporophyte fossils (see here). Early in their history, land plants also evolved a cuticle. The cuticle is a waxy layer on the outer surface of the aerial parts of a plant that helps to prevent water loss. Sometimes cuticle with details of epidermal cell structure is preserved in fossil plants.
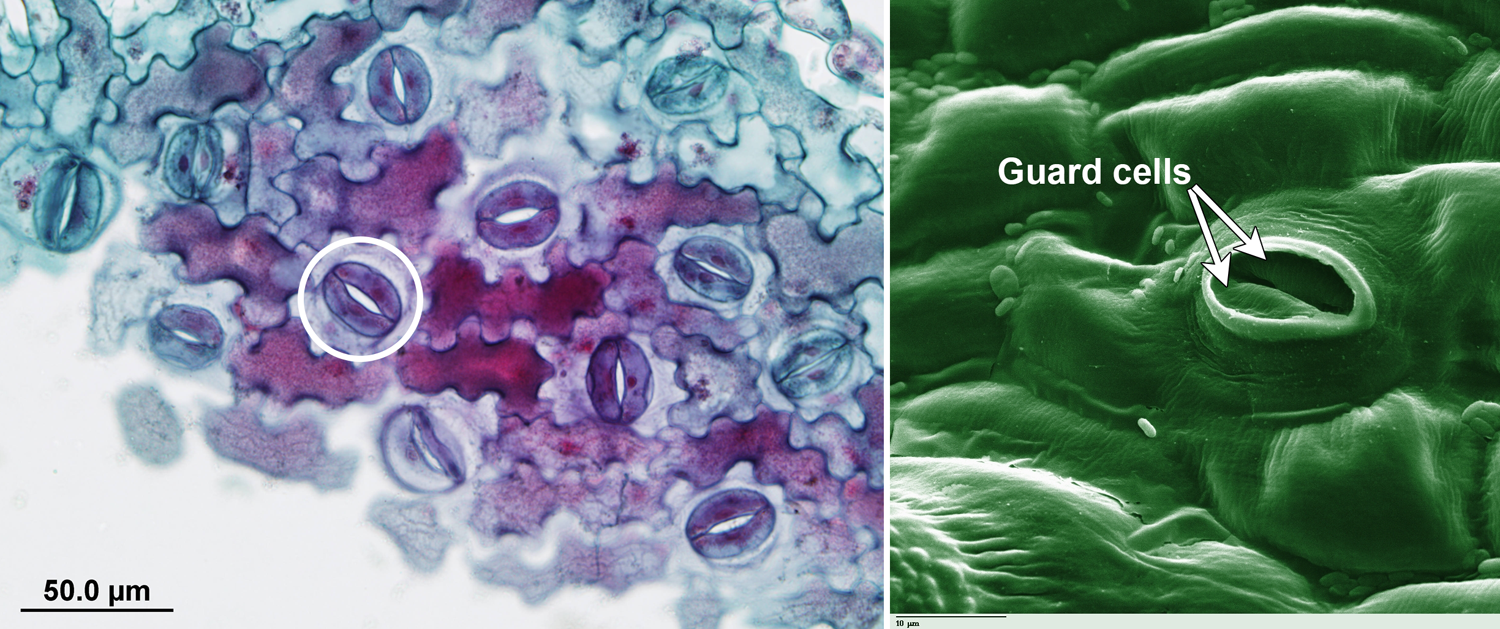
Examples of stomata in modern angiosperms. Left: Paradermal section of the epidermis (outer tissue layer) of wood-oil tree (Aleurites montana) showing stomatal apparatuses (pores surrounded by guard cells) and puzzle-shaped epidermal cells. An example of a stomatal apparatus is circled. Right: Scanning electron photomicrograph of tomato (Solanum lycoperiscum) showing stoma with guard cells on epidermis (false color image). Credits: Aleurites montana paradermal section (via Swingle Plant Anatomy Collection Digital Archive, University of Miami, Public Domain in the United States); tomato leaf stomate (Photohound, via Wikimedia Commons, Public Domain).
The first structurally informative sporophyte macrofossils represent the sporophytes of polysporangiophytes ("many-sporangium plants"), which are first known from the Silurian period. In polysporangiophytes, the sporophyte axis (stalk) branches and more than one sporangium (spore-producing capsule) is produced. Most living plants are polysporangiophytes. At least some of these early branching sporophytes would have become capable of sustaining themselves after a period of dependence on the gametophyte. At some point, the sporophytes of polysporangiophytes began to exhibit indeterminate growth. This means that a single sporophyte can grow and elongate indefinitely from specialized growing regions (meristems) at the tips of its axes.
All living and most extinct polysporangiophytes are also tracheophytes (vascular plants). The sporophytes of tracheophytes have specialized conducting tissues (vascular tissues) to shuttle food and water through the plant body. Phloem is the tissue that transports food, whereas xylem is the tissue that transports water. The origination of xylem in the fossil record is signified by the appearance of the tracheid, a type of water-conducting cell. Tracheids are also first recorded in the Silurian, although they are inferred to have originated after the evolution of the branching sporophyte.

Cooksonia, an early polysporangiophyte and tracheophyte (Late Silurian). Left (A-B). Branching axes of Cooksonia pertoni from the Late Silurian of the UK. Note terminal sporangia. (scale bar = 2 mm). Right. Reconstruction of a Cooksonia sporophyte. Credits: Cooksonia pertoni fossils (from Figure 2 in Edwards & Kendrick 2015 Philos Trans, Ser B, CC BY 4.0); Cooksonia model (MUSE-Science Museum of Trento, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
The story of land plant structural evolution is the story of the evolution and elaboration of the sporophyte generation. After attaining indeterminate apical growth, apical branching, and vascular tissue, sporophytes continued to become more complex. Some innovations include the origination of different types of branching, organs (leaves, roots), wood, and specialized reproductive structures (cones, flowers). It should be noted that many sporophyte structural innovations have arisen more than once in land plants. For example, leaves, cones, and the arborescent growth habit (tree-type form) have evolved separately in unrelated groups. Thus, the evolution of the sporophyte is not a story of simple, linear progression, but is instead a process of repetitive structural evolution and innovation.
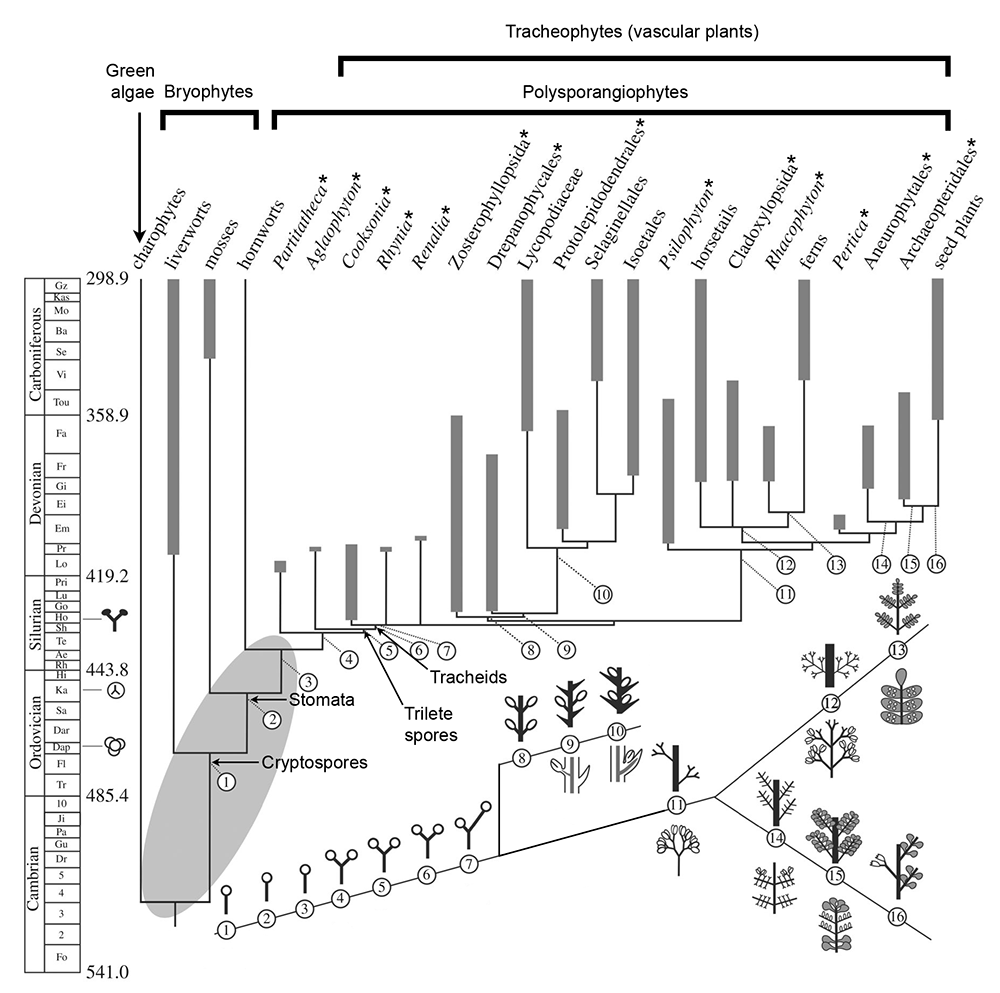
Key innovations in the structure of the land plant sporophyte during the Paleozoic. This diagram shows a hypothetical phylogeny for land plants scaled to time. Extinct taxa are marked by an asterisk (*). Major groups of organisms represented include the green algae (represented by charophytes), bryophytes (liverworts, mosses, and hornworts), polysporangiophytes (everything from Partitatheca to seed plants on the tree), and tracheophytes/vascular plants (Cooksonia to seed plants on the tree). A portion of the Geologic Time Scale is shown on the left, with the numerical ages of boundaries between time periods marked in millions of years before present. Fossil evidence for land plants (bottom to top: cryptospores, trilete spores, and sporophytes) is marked on the time scale. Thick gray lines = observed stratigraphic range; thin black lines = inferred range.
Key innovations inferred to have occurred in the structure of the land plant sporophyte are shown underneath the phylogenetic tree. The number for each innovation is mapped to its position on the phylogenetic tree. 1-3. Unbranched sporophyte with terminal sporangium (spore capsule). 4-6. Sporophyte with isotomous (equal) apical branching. 7. Sporophyte with unequal apical branching. 8-10. Evolution of leaves and lateral sporangia in the lycophyte lineage. 11-16. Evolution of branching, leaves, and sporangial position in the euphyllophyte lineage (including horsetails, ferns, seed plants, and extinct relatives). Figure caption adapted from and image modified from Figure 1 (CC BY 4.0) in Harrison & Morris (2017).
Selected references & additional reading
Note: Free full text is made available by the publisher for items marked with a green star.
Academic articles & book chapters
Blackwell, W.H. 2003. Two theories of the land-plant sporophyte: Which is left standing? Botanical Review 69: 125-148. https://doi.org/10.1663/0006-8101(2003)069[0125:TTOOOT]2.0.CO;2
* Doyle, J.A. 2013 [republished online 2017]. Phylogenetic analyses and morphological innovations in land plants. In: B.A. Ambrose and M. Purugganan, eds. The Evolution of Plant Form. Annual Plant Reviews 45. https://doi.org/10.1002/9781119312994.apr0486
* Edwards, D., and P. Kendrick. 2015. The early evolution of land plants, from fossils to genomics: a commentary on Lang (1937) 'On the plant-remains from the Downtonian of England and Wales.'Philosophical Transactions of the Royal Society B, Biological Sciences 370: 20140343. http://dx.doi.org/10.1098/rstb.2014.0343
* Falcón, L.I., S. Magallón, and A. Castillo. 2010. Dating the cynaobacterial ancestor of the chloroplast. The ISME Journal 4: 777-783. https://doi.org/10.1038/ismej.2010.2
* Finet, C., R.E. Timme, C.F. Delwiche, and F. Marlétaz. 2010. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Current Biology 20: 2217-2222. https://doi.org/10.1016/j.cub.2010.11.035
* Graham, L.E., M.E. Cook, and J.S. Busse. 2000. The origin of plants: Body plan changes contributing to a major evolutionary radiation. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.97.9.4535
* Harrison, C.J., and J.L. Morris. 2017. The origin and early evolution of vascular plant shoot and leaves. Philosophical Transactions of the Royal Society B, Biological Sciences 373: 20160496. https://dx.doi.org/10.1098/rstb.2016.0496
* Keeling, P.J. 2004. Diversity and evolutionary history of plastids and their hosts. American Journal of Botany 91: 1481-1493. https://doi.org/10.3732/ajb.91.10.1481
Keeling, P.J. 2013. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annual Review of Plant Biology 64: 583-607. https://doi.org/10.1146/annurev-arplant-050312-120144
* Ligrone, R., J.G. Duckett, and K.S. Renzaglia. 2012. Major transitions in the evolution of early land plants: a bryological perspective. Annals of Botany 109: 851-871. https://doi.org/10.1093/aob/mcs017
* Morris, J.L., M.N. Puttick, J.W. Clark, D. Edwards, P. Kenrick, S. Pressel, C.H. Wellman, Z. Yang, H. Schneider, and P.C.J. Donoghue. 2018. The timescale of early land plant evolution. Proceedings of the National Academy of Sciences U.S.A. 115: E2274-E2283. https://doi.org/10.1073/pnas.1719588115
* Niklas, K.J., and U. Kutschera. 2010. The evolution of the land plant life cycle. New Phytologist 185: 27-41. https://doi.org/10.1111/j.1469-8137.2009.03054.x
Punt, W., P.P. Hoen, S. Blackmore, S. Nisson, and A. Le Thomas. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1-81. https://doi.org/10.1016/j.revpalbo.2006.06.008
* Rubinstein, C.V., P. Gerrienne, G.S. de la Puente, R.A. Astini, and P. Steemans. 2010. Early Middle Ordovician evidence for land plants in Argentina (eastern Gondwana). New Phytologist 188: 365-369. https://doi.org/10.1111/j.1469-8137.2010.03433.x
* Ruggiero, M.A., D.P. Gordon, T.M. Orrell, N. Bailly, T. Bourgoin, R.C. Brusca, T. Cavalier-Smith, M.D. Guiry, and P.M. Kirk. 2015. A higher level classification of all living organisms. PLoS ONE 10(4): e0119248. https://doi.org/10.1371/journal.pone.0119248
Spiegel, F.W. 2012. Contemplating the first plantae. Science 335: 809-810. https://doi.org/10.1126/science.1218515
Steemans, P., E. Petus, P. Breuer, P. Mauller-Mendlowicz, and P. Gerrienne. 2012. Palaeozoic innovations in the micro- and megafossil plant record: from the earliest plant spores to the earliest seeds. Pgs. 437-477 in J.A. Talent, ed. Earth and Life, International Year of Planet Earth. Springer Science+Business Media. https://doi.org/10.1007/978-90-481-3428-1_13
Strother, P.K. 2000. Cryptospores: The origin and early evolution of the terrestrial flora. In: R.A. Gastaldo and W.A. DiMichele, eds. Phanerozoic Terrestrial Ecosystems. The Paleontological Society Papers 6: 3-20.
Strother, P.K., and W.A. Taylor. 2018. The evolutionary origin of the plant spore in relation to the antithetic origin of the plant sporophyte. Pgs. 3-20 in: M. Krings, C.J. Harper, N.R. Cúneo, and G.W. Rothwell, Transformative Paleobotany, papers to commemorate the life and legacy of Thomas N. Taylor. Academic Press, London, UK. https://doi.org/10.1016/B978-0-12-813012-4.00001-2
* Wagner, S.C. 2011. Biological nitrogen fixation. Nature Eduction Knowledge 3(10): 15. https://www.nature.com/scitable/knowledge/library/biological-nitrogen-fixation-23570419/
Wellman, C.H., P.L. Osterloff, and U. Mohiuddin. 2003. Fragments of the earliest land plants. Nature 425: 282-285. https://doi.org/10.1038/nature01884 PDF reprint
Books & textbooks
Cooke, M.C. 1882-1884. British fresh-water algae, exclusive of Desmidieae and Diatomaceae, vol. II. Plates. Williams and Norgate, London, Edinburgh. PDF from Internet Archive.
Evert, R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York.
Simpson, M.G. 2010. Plant Systematics, 2nd ed. Academic Press, Burlington, Massachusetts.
Taylor, T.N., E.L. Taylor, and M. Krings. 2009. Paleobotany, The Biology and Evolution of Fossil Plants, 2nd ed. Academic Press, London.
Websites
APSA Members. 2007. The Australasian Pollen and Spore Atlas V1.0. Australian National University, Canberra. http://apsa.anu.edu.au/
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
Page first released: 30 August 2019; last updated: 13 June 2020.