Chapter contents:
Paleoecology
– 1. Paleoenvironmental Reconstruction
– 2. Biogeochemical Analysis and Paleoecology
– 3. Predation in Paleoecology
–– 3.1 Insect Herbivory in the Fossil Record
–– 3.2 Drilling Predation in the Fossil Record ←
–– 3.3 Dinosaur Predation in the Fossil Record
Above image by Jansen Smith.
Overview
When examining interactions between individuals in the fossil record, drilling predation on marine invertebrates is among the most commonly studied due to the widespread distribution of marine invertebrates in the fossil record and the ease with which drill holes can be identified. This type of predation is named for the characteristic trace fossil that is left behind: a drill hole. Drill holes can be made by a variety of predatory snails in the families Cassidae, Muricidae, and Naticidae, and also by some octopus. For this section, we will focus primarily on predation by naticids, which are also called moon snails.

A naticid specimen of the species Neverita didyma. The brown tissue on which the shell appears to be sitting is the snail's foot. Image by Almandine; Creative Commons Attribution-Share Alike 3.0 Unported, 2.5 Generic, 2.0 Generic and 1.0 Generic licenses.
Fossil shell of the naticid snail Naticarius plicatella from the Pliocene of Sarasota County, Florida (PRI 70745).
Drillhole in the shell of the oyster Ostrea coxi. This specimen is from the Plio-Pleistocene of Sarasota County, Florida (PRI 40844).
How is a drill hole made?
Naticids first evolved at least 200 million years ago, with many species alive today, and it is likely they used the drilling behavior for all or most of their evolutionary history. If you have ever gone to the beach, odds are you have seen naticid seashells and it is even more likely that you have seen their handiwork (i.e., drill holes) in other shells on the beach. This is likely to be the case because naticids are found around the world in habitats close to the shore, although some species live deeper in the ocean. Quite often, naticids can be found at low tide—when they are exposed to the air—while they are hunting other snails and clams. You might not see the snail at first, but if you follow one of their trails in the sand, there will probably be a snail at the end of it.
If the naticid was successful in its hunt, you might find it holding onto another snail or clam in its muscular foot. In addition to being used for movement, the snail’s foot is also what it uses to capture and subdue its prey. As you can see in the video below, it is not always easy to get a hold of a clam! Once it has been captured, the clam (or snail) is held tightly in the foot and the naticid will often burrow into the ocean floor to hide while it undertakes the process of drilling a hole in the shell.
"Molluscs: Moon Snail Preys on Cockles" by Shape of Life. Source: shapeoflife.org
To drill a hole, naticids use a combination of physical scraping and acid application to remove a perfectly circular section of the shell. Naticids alternate between acid and scraping throughout the process. The acid eats away the shell and softens it. Scraping, with a rasping tongue-like tool called a radula (watch the video below to see a snail radula in action), removes the softened shell. Depending on the thickness of the shell being drilled, this process can take dozens of hours to complete. After completing the hole, the naticid secretes an acid into the opening, which will externally digest the contents of the clam or snail it is eating. Once it has been sufficiently broken down, the naticid inserts a straw-like appendage, called a proboscis, into the shell to consume its meal.
Although this is an harbivorous snail, its radula performs a similar function to that of a predatory naticid. "Trochus snail feeding" by Digital Atlas of Ancient Life. Source: YouTube.
Who made the drill hole?
Now that you know a little more about the process of how a drill hole is formed, we will take a look at how drill holes made by members of different groups (e.g., naticids versus muricids) are identified. Many of the drill holes found in the fossil record are made by either a naticid or muricid snail.
Ecphora, an extinct type of muricid snail. This specimen is from the Pliocene of Hampton County, Virginia (PRI 70750).
Although muricids tend to have more ornate shells and are a much more diverse group than naticids, the drilling process is fairly similar. When it comes to the shape of the drill hole, however, there is one major difference: muricid drill holes tend to be straight-sided and naticid drill holes tend to have beveled sides. Look at the figure below. Which drill holes do you think were made by a muricid? A naticid? To check, click on the dropdown in the figure caption. The shape of the drill hole is a good indicator in many cases but is not as reliable when the shell of the prey is relatively thin. When the shell is thin, the characteristic shapes of the naticid and muricid drill holes look very similar. Depending on the predator species, drill holes made by muricids can also be identified based on the size of the drill hole. Muricids tend to make smaller drill holes than naticids.

Based on observations of the scraping patterns made by the radula of naticid and muricid snails during the drilling process, it has been proposed that these patterns could be used to identify the predator who made the hole. The initial studies of these trace fossils took place decades ago and the idea has recently been revisited. The use of these patterns is an intriguing idea but is complicated in practice. In order to use the scraping patterns, specific patterns must first be unequivocally linked to a specific predator. This link may be possible through experimentation with live animals—and would require the assumption of taxonomic uniformitarianism for application to the fossil record.
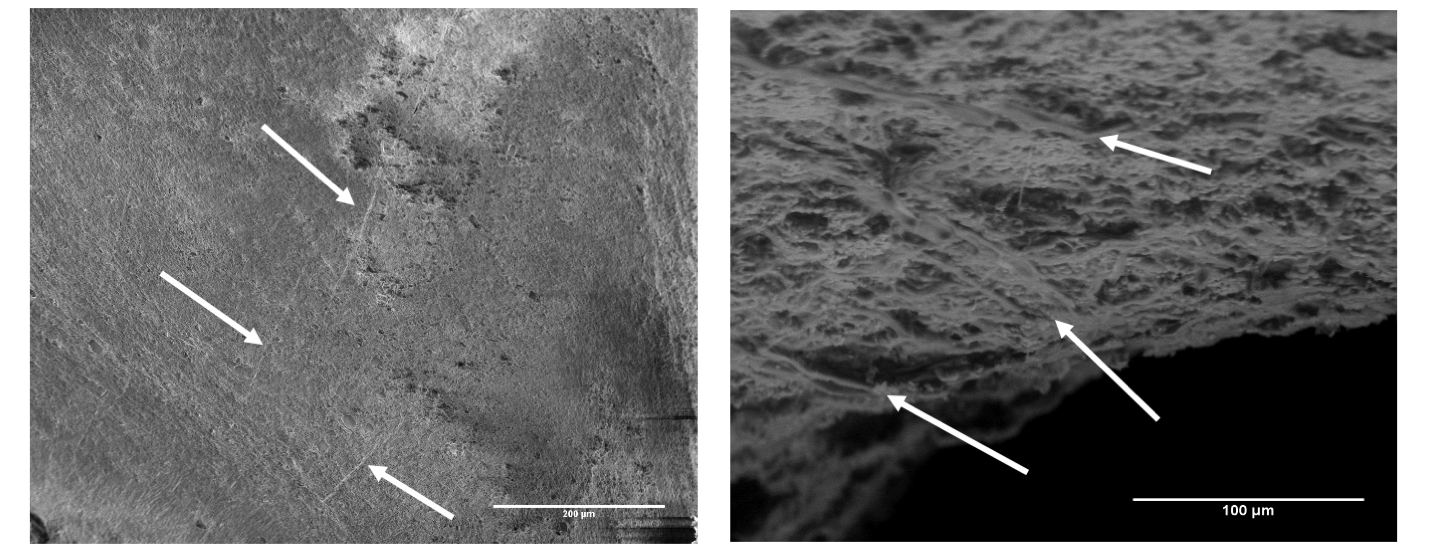
Scanning electron microscope images showing potential radula scrape marks (white arrows) from the drilling process. The left image is a trace from the naticid Euspira on a mussel, Mytilus. The right image is a trace from a Euspira on a Littorina snail.
Identification based on the patterns is also confounded by the alternating process of scraping and acid use by the drilling snails. Scraping is only a part of the drill hole formation process and, even if proven to be diagnostic, the use of acid by the snail in between periods of scraping may remove the scrape marks. Finally, the scraping patterns must preserve in the fossil record. As discussed previously, taphonomic processes commonly alter organismal remains, potentially including any existing scrape marks. Even though it is possible for many things to “go wrong” in using radula scraping patterns, if it can be proven that they are a reliable indicator of predator identity, it would open new lines of research in the study of drilling predation in the fossil record and enable much greater specificity in the questions paleoecologists ask.
What can we learn from drill holes?
Drill holes have been used to address a variety of research questions in paleoecology. These have included, and are not limited to: how frequently was one species eaten compared to another? What is the biggest risk of death for a given species (where drilling predation is only one risk)? Do naticids have a favorite type of prey? And, has the frequency of predation changed over time? In answering these questions, paleoecologists commonly make use of the assumption of taxonomic uniformitarianism and, whenever studying more than one individual, also employ the assumption that fossil assemblages represent real communities. As we consider how drill holes are studied, keep in mind that these assumptions underlie most paleoecological studies.
Cannibalism
In addition to eating snails and clams of different species, naticids are also known to eat other naticids, including those of the same species. That is, many naticid species are cannibals! This propensity to consume one another can lead to complicated ecological dynamics and has also served to confuse researchers early in the study of naticid predation. Indeed, the first explanation of naticid cannibalism was simply that the naticids were inept predators and were unable to distinguish a conspecific—belonging to the same species—from an individual of a different species. This idea has subsequently fallen from favor after comparisons with other cannibalistic species, experimental studies with live naticids, and observations of naticid drilling predation in the fossil record. Considering other cannibal species (read more about cannibalism here), several factors tend to explain the behavior. First, there is almost always competition for resources, like food and mates, in nature and cannibalism is an excellent way to eliminate the competition. Second, eating a conspecific not only removes a competitor, it is also a valuable food source. From an energetics standpoint, eating a conspecific can actually be more beneficial compared to eating an individual of a different species. Third, cannibalism can be a voluntary affair. For some species, a mate or a parent may allow itself to be consumed to provide nutrients to its reproductive partner or offspring. Ultimately, the extra nutrients from the consumed individual can help increase the odds that its offspring survive and reproduce. Although it remains possible that ineptitude could play a role, these explanations are more biologically plausible.

Naticid snails (Neverita reclusiana) from the northern Gulf of California. Several of the Neverita show bevelled drill holes in their shells, suggesting they were cannibalized. Note that only the smaller individuals have drill holes, as larger individuals tend to prey on smaller individuals in this interaction. Photograph by Jansen A. Smith.
Experimental studies on living naticids have further supported the notion that cannibalism likely occurs intentionally. Namely, naticids have been shown to “choose” their prey according to the net energy they derive from it; this is also referred to as a cost-benefit ratio. In a controlled laboratory setting, a research team led by Jennifer Kitchell experimented with naticid choice of prey. When presented with multiple different types of prey, each with known and different cost-benefit ratios, Kitchell and her colleagues found that the naticids would predictably choose the types of prey with greater energetic value. Although they did not present naticids with the opportunity to cannibalize in their experiments, when the energetic value of a naticid is compared to the prey types that were presented to the naticid predator, the naticid prey had an equally-high value. This suggests that, from a purely energetic standpoint, naticids could stand to benefit from eating each other.
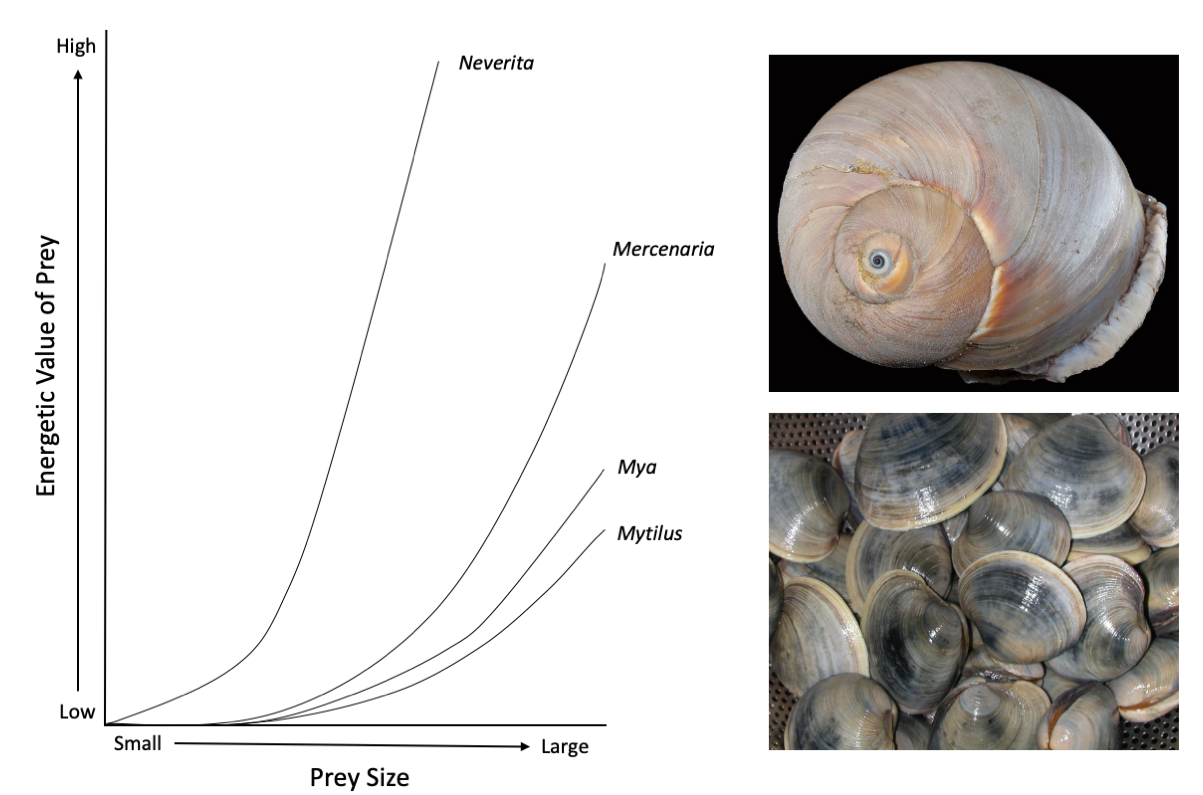
The left image shows the energetic value of different prey, at different sizes. Neverita, the naticid pictured in the top left, has a higher energetic value (as a prey item) than several of the clams commonly eaten by naticids like Mercenaria (bottom right), Mya, and Mytilus. The left image is redrawn from Kitchell et al. (1981) in Paleobiology. Top right image by Smithsonian Environmental Research Center; Creative Commons Attribution 2.0 Generic license. Bottom right image by 本人; Creative Commons Attribution-Share Alike 3.0 Unported license.
With these experimental studies as a backdrop, observational studies of naticid predation in the fossil record have supported the idea that naticids are selective and stereotypical in their predation. In a collection of studies by Patricia Kelley, naticids were shown to consistently place their drill holes at approximately the same location on their prey shells. This was true not only for clam species they consumed, but also when the naticids cannibalized. This repeatable placement suggests an effective predator rather than ineptitude. With all the comparative, experimental, and observational evidence, it is now clear that cannibalism in naticids does not occur as an error.
Drilling frequency through time
Most studies of naticid predation include some calculation of drill hole frequency in prey species. Drilling frequency is a relatively simple measure—calculated as the number of drilled individuals divided by the total number of individuals in the assemblage—and there has been a temptation to evaluate changes in drilling frequency over time, including across such wide spans of time as the Cenozoic (i.e., approximately the last 66 million years), and even through the entire Phanerozoic (i.e., approximately the last 541 million years). Based on what you have already read in this chapter, what types of issues do you think might arise when making comparisons over these long timespans?
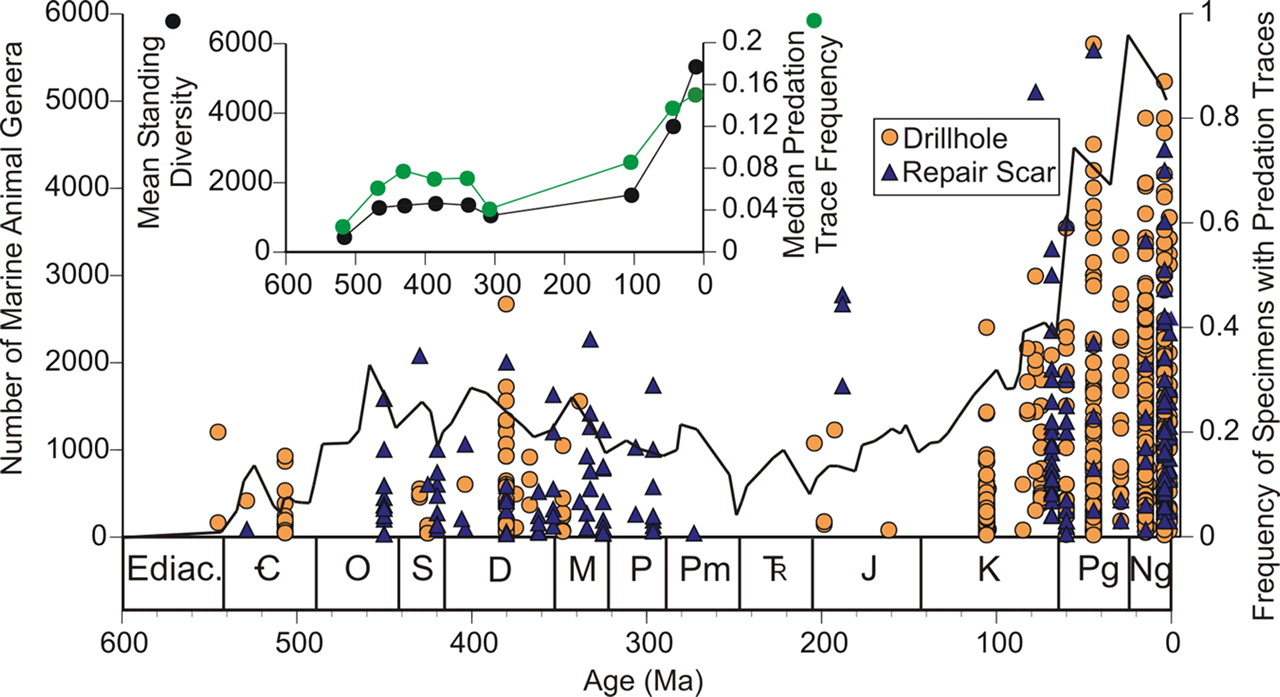
"Phanerozoic history of genus-level marine animal diversity (black line) (22) and predation trace frequency of marine invertebrates (orange circle, drillhole; blue triangle, repair scar). (Inset) Average diversity (black circles) and predation (green circles) values for each period with at least 10 predation occurrences" Image and caption from Huntley and Kowalewski (2007) in Proceedings of the National Academy of Sciences under the PNAS License to Publish (Copyright (2007) National Academy of Sciences).
As discussed earlier in this chapter, scientific studies are strongest and most compelling when the number of assumptions they make is limited. When comparing drilling frequencies over long time spans, the number of assumptions required to make such comparisons is relatively high. This is not to say that the results of these studies (e.g., an increase in drilling frequency moving from past to present) are erroneous, but does mean that they merit close examination. Studies that make comparisons over long timespans tend to be based on data that are aggregated from the existing scientific literature. This means there is little control over the quality of the data, including such issues as sampling procedure, sample processing, and identification. Most studies will contain reliable data; however, small between-study differences have the potential to increase the variability in the combined dataset and decrease the strength of conclusions from the compiled data.
Additionally, and more importantly, there is often no control for the habitat from which data originated, meaning one dataset could be from a temperate estuary, another from a tropical continental shelf, and a third from a polar archipelago. As we have learned from the study of modern organisms, and even in fossil studies, predator-prey interactions are influenced by the environment in which the organisms are interacting. These influencing factors include abundance of other naticids, availability and abundance of prey, abundance of predators (e.g., crabs, birds, fish) of naticids, water temperature, water chemistry, and many more factors that vary on relatively small geographic scales. Most studies control for some of these factors and, for the few they cannot control, assumptions get made. In contrast, studies on long time scales with data compiled from other scientific studies routinely do not control for any of these factors that might influence drilling frequencies over time. Though it remains possible that these factors balance each other out over long timespans (e.g., tens and hundreds of millions of years), much work remains to do done to verify the patterns reported in these studies of drilling frequency on long timespans.
Predation in a community context
Studies of naticid predation have traditionally compared the frequency of predation on a particular species to frequencies on other species in the community, or on the same species at a different point in time. These studies can provide information on which species was more preferred by the predator, whether the preference for a certain species changed over time, and how frequently predation occurred in the community as a whole. Paleoecologists can use this information on the preferences of a predator to interpret ecological and evolutionary patterns in the fossil record.
When drawing conclusions from drilling predation data, it can be informative to make comparisons to a null hypothesis—a default expectation that there is no difference between species or communities being compared—because it provides a standardized basis for comparison. For example, if upon examining a fossil community, you found that clam species A was drilled at a frequency of 10% and clam species B was drilled at 14%, what could you conclude about the preference for these two clams? You could say that clam B is preferred relative to clam A, after all, it has a higher drilling frequency. But, you do not know if either clam was actually preferred in general. At the community level, if the average drilling frequency was 14%, you might conclude that neither clam A nor clam B was preferred because neither is drilled at an above average frequency. By comparison to this null for the community, you might even conclude that the naticid predators actually avoided eating clam A.
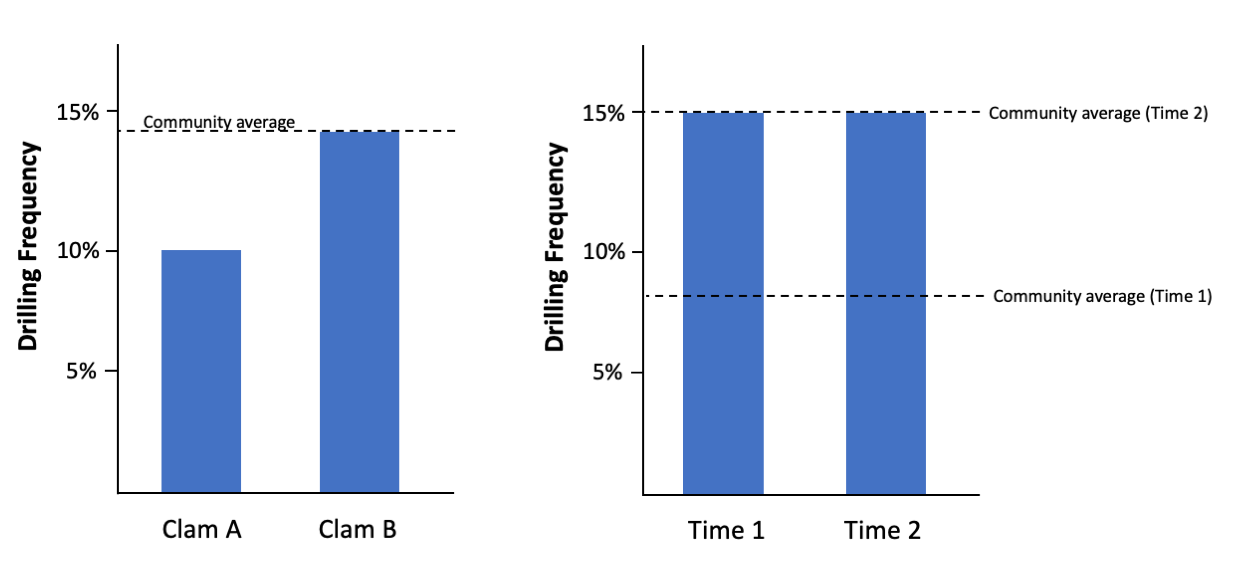
In the figure on the left, clam species B appears to be preferred over clam species A by the naticid predator; however, compared to the community-wide frequency, neither clam is preferred overall. In the figure on the right, clam species C is drilled at the same frequency at times 1 and 2; however, the community frequency changed from time 1 to time 2. At time 1, the community average was lower, and the clam was preferred by comparison. At time 2, the community average increased and the clam was no longer preferred. Figure by Jansen Smith.
We can also consider the importance of this null hypothesis when assessing drilling frequency on a species at different time intervals. In this case, we will say clam species C was found at time 1 and time 2, and 15% of individuals were drilled at each time interval. Without comparing to the null frequency for the community, what conclusion would you draw for the preference of naticid for clam C in time 1 and time 2? What if the null of the community was 8% during time 1 and rose to 15% during time 2? Through this example, you can readily see how your interpretation might change. Even though drilling frequency remained the same for clam C, comparison to the community null shows that it was preferred during time 1, but not during time 2. The reason for this change in preference between time 1 and 2 could relate to a variety of factors like the abundance of naticids in the community, availability and abundance of other types of prey, abundance of predators (e.g., crabs, birds, fish, or naticids), water temperature, or water chemistry. Attempting to explain why the difference exists can be challenging and is often the focus of paleoecological studies; however, it is important to compare first to a null comparison to provide context for the interaction within the broader community.
An example from the Colorado River estuary
For a real-world example, we will turn again to the Colorado River estuary. In this estuary, the seashore is lined with unique assemblages of seashells that were deposited over the last several thousand years. Examination of these assemblages shows that one clam, Mulinia modesta, was extremely abundant in the past and that it was commonly consumed by the naticid, Neverita reclusiana, in the past. After the damming of the Colorado River in the early twentieth century, the population of Mulinia declined. Because Mulinia was potentially an important part of the diet for Neverita, it was hypothesized that the population of Neverita may also have declined through loss of food. Alternatively, the Neverita in the estuary may have been able to switch to eating a different kind of clam.
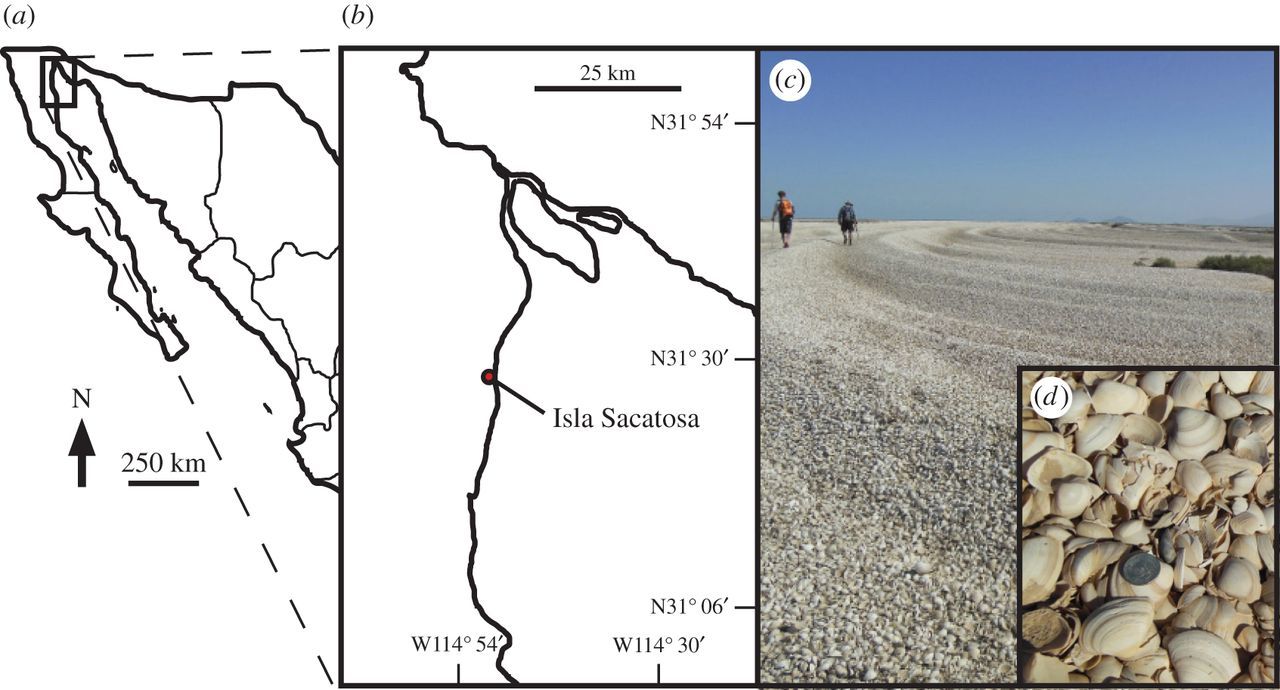
"Cheniers in the Colorado River delta. (a) Location of delta in Mexico. (b) Colorado River delta locality from Kowalewski et al. (2000). (c) A chenier in the Colorado River delta at a locality south of Isla Sacatosa. (d) Close-up of Mulinia coloradoensis; coin, 0.24 cm in diameter." Image and caption from Smith et al. (2016) in Royal Society Open Science; Creative Commons Attribution 4.0 International license.
To distinguish between these hypotheses, researchers Jansen Smith, Gregory Dietl, and John Handley examined the preference of Neverita for different species in the past. The researchers found that, although Mulinia was a highly preferred prey species of Neverita in the northern part of the estuary, Mulinia was not as highly preferred as other prey species in the southern portion of the estuary. Interestingly, the drilling frequency on Mulinia did increase moving south, but the increase in drilling frequency was small compared to changes for other species in the community. Without comparison to a null hypothesis, it would have appeared as if Mulinia was actually more preferred in the south—a faulty conclusion. In the past, the northern estuary was more strongly influenced by freshwater flow from the Colorado River and the southern estuary was more similar to the normal marine conditions in the Gulf of California. Today, as the northern estuary is becoming more like the rest of the Gulf, species from the southern estuary are moving northward. Thus, it is highly likely that Neverita does have alternative prey species to eat, even with the decline in the Mulinia population.

A specimen of Neverita reclusiana and Mulinia modesta. Photographs by Jansen Smith.
Concept check: See what you know!
Describe the process undertaken by a naticid to drill a hole and consume its prey.
After subduing its prey using its muscular foot, a naticid alternates between excreting acid on the shell and using its radula to scrape away the softened shell. Following the formation of a hole, the naticid secretes a digestive acid into the shell of its prey, then consumes the liquified tissue using its proboscis.
Name one major difference between the drill holes produced by naticid and muricid snails.
The major difference is in the shape of the drill hole. Naticids tend to produce beveled holes, which start wider and narrow during the drilling process. In contrast, muricids tend to produce straight-sided holes.
True or False: Cannibalistic drill holes made by one naticid on another arise through ineptitude by the predator.
False! Though it was once thought that this was true, more recent evidence suggests that cannibalism in naticids is a beneficial behavior, particularly in competitive conditions.
What factors can influence the behavior of, and drilling frequencies from, naticids?
Many factors can influence the drilling behavior including, but not limited to, abundance of other naticids, availability and abundance of prey, abundance of predators (e.g., crabs, birds, fish) of naticids, water temperature, and water chemistry.
Why is it important to assess drilling frequencies within a community context?
Community context provides additional information that can be used to interpret patterns in drilling frequency. A high drilling frequency could result from a strong preference for a particular prey, or simply because drilling in the whole community was high. Knowing the difference is key!
References and Further Reading
Huntley, J. W., and M. Kowalewski. 2007. Strong coupling of predation intensity and diversity in the Phanerozoic fossil record. Proceedings of the National Academy of Sciences, 104: 15006-15010.
Kelley, P. H. 1988. Predation by Miocene gastropods of the Chesapeake Group: stereotyped and predictable. Palaios, 3: 436-448.
Kelley, P. H. 1991. Apparent cannibalism by Chesapeake Group naticid gastropods: a predictable result of selective predation. Journal of Paleontology, 65: 75-79.
Kitchell, J. A., C. H. Boggs, J. F. Kitchell, and J. A. Rice. 1981. Prey selection by naticid gastropods: experimental tests and application to the fossil record. Paleobiology, 7: 533-552.
Kowalewski, M. 1993. Morphometric analysis of predatory drillholes. Palaeogeography, Palaeoclimatology, Palaeoecology, 102: 69-88.
Schiffbauer, J. D., Y. Yanes, C. L. Tyler, M. Kowalewski, and L. Leighton. 2008. The microstructural record of predation: a new approach for identifying predatory drill holes. Palaios, 23: 810-820.
Smith, J. A., J. C. Handley, and G. P. Dietl. 2018. On drilling frequency and Manly's alpha: towards a null model for predator preference in paleoecology. Palaios, 33: 61-68.
Smith, J. A., J. C. Handley, and G. P. Dietl. 2018. Effects of dams on downstream molluscan predator–prey interactions in the Colorado River estuary. Proceedings of the Royal Society B: Biological Sciences, 285: 20180724.
Tyler, C. L., and J. D. Schiffbauer. 2012. The fidelity of microstructural drilling predation traces to gastropod radula morphology: paleoecological applications. Palaios, 27: 658-666.
Usage
Unless otherwise indicated, the written and visual content on this page is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 International License. This page was written by Jansen A. Smith. See captions of individual images for attributions. See original source material for licenses associated with video and/or 3D model content.




