Chapter contents:
Echinodermata
–– 1. Exclusively Fossil Taxa
–– 2. Crinoidea ←
–– 3. Asteroidea
–– 4. Ophiuroidea
–– 5. Echinoidea
–– 6. Holothuroidea
You can find 3D models of Crinoidea here!
This page was written by Jansen Smith. It was last updated on May 26, 2020.
Above image: Fossil crinoids from the Jurassic by Kevin Walsh; Creative Commons Attribution 2.0 Generic license.
Class Crinoidea Snapshot
- Examples: sea lillies and feather stars
- Ecology: marine filter feeders
- Key features of group: pinnuled arms, multi-component stalk
- Diversity: ~660 living sp., ~6,000 extinct sp.
- Fossil record: Ordovician to Recent
Overview
Crinoids, like other members of the phylum Echinodermata, are exclusively marine animals with pentaradial symmetry and water-vascular systems. Though some groups have lost the stalk in adult forms, crinoids are considered to follow the stalked, radial morphology, as the stalkless forms are derived from stalked ancestors. Crinoids are generally considered to be the sister group (i.e., closely related but least similar) to the other living echinoderm classes (see the phylogeny below). Unique from other extant echinoderm classes, crinoids feed primarily by filter feeding. To feed, crinoids use their stalk, or column, to elevate the crown (i.e., cup with vital organs, and feather-like arms) into the water column. When the stalk is present, as in most fossil forms, crinoids are often referred to as sea lilies—crinoid means "lily-like" in Greek. The stalk has been lost in adults of many modern crinoids (a stalk is present in larval stages), called feather stars, as an adaptation to be more mobile than their fossil predescessors. Today, more than 660 species of living crinoid have been identified, and more than 6,000 fossil species have been described, with the oldest dating to the Tremadocian Stage (485.4 – 477.7 million years ago) of Ordovician Period. In the sections that follow, we will explore the anatomy of crinoids before looking at their diversity and ecology from the Ordovician to the present.
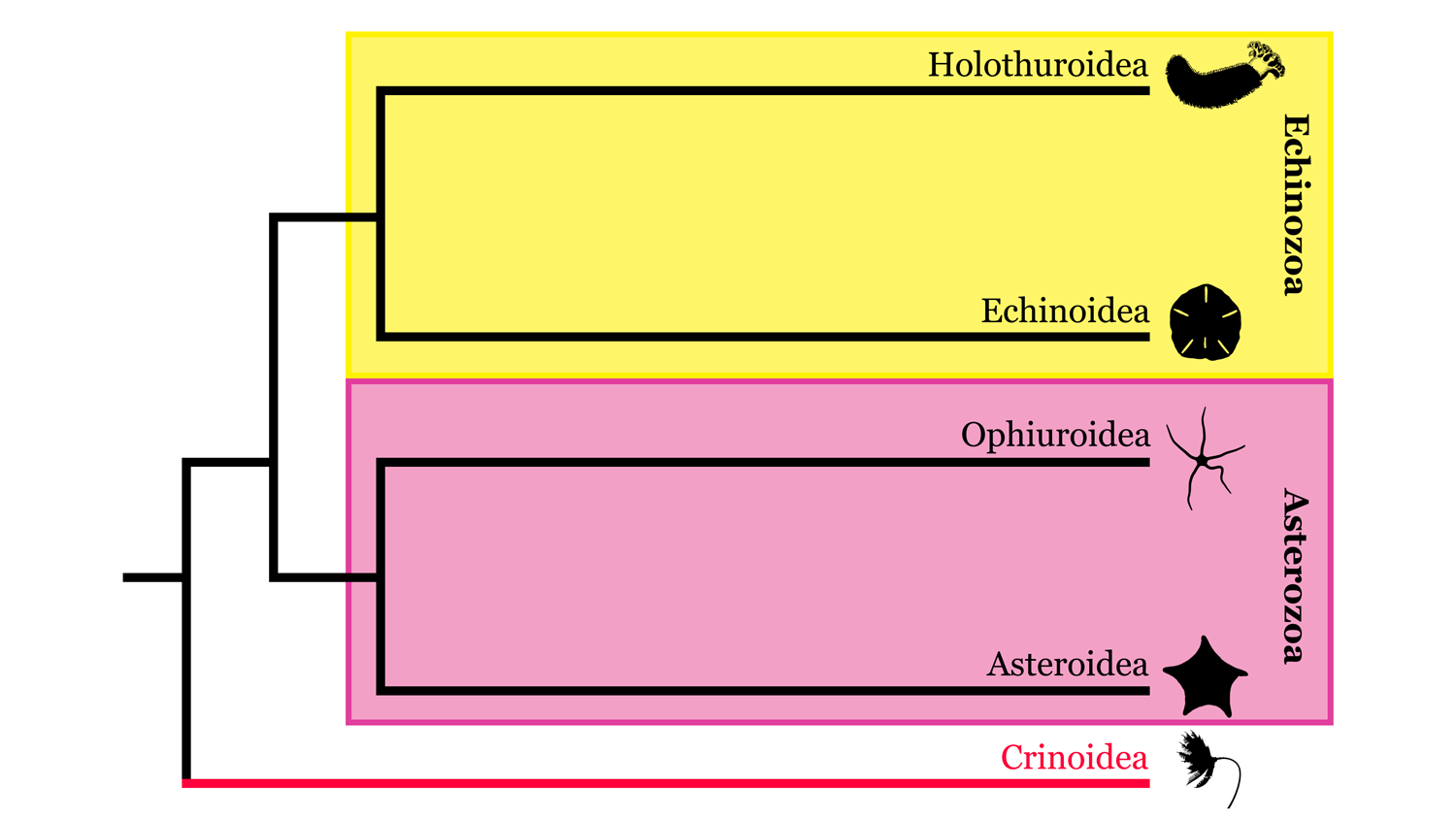
Highly simplified overview of Echinodermata phylogeny based in part on the hypothesis of relationships presented by Reich et al. (2015). Image by Jaleigh Q. Pier; Creative Commons Attribution-Share Alike 4.0 International license.
Crinoid skeletal anatomy
The crinoid skeleton contains numerous elements made of magnesium-rich calcite and are held together by a combination of ligaments and muscles. Crinoids have two general components, a crown used for feeding and reproduction, and a column used to elevate the crown into the water column and for attachment to the ocean bottom. The crown and stalk each include two parts. The crown includes arms, which attach at their base in a radial arrangement to the second part, a cup or a ovate calyx, which contains the vital organs. The two parts of the stalk are the columnals, which give the crinoid its height, and the holdfast, which is used to keep the crinoid in place. In the following sections, we will take a closer look at each of these parts.
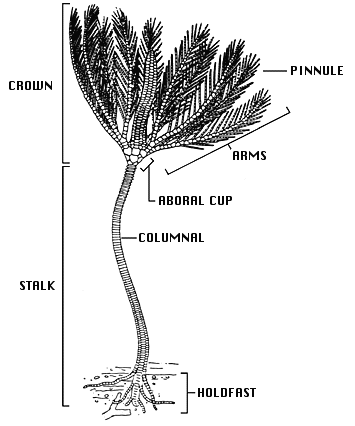
Basic anatomy of a crinoid. Image by William I. Ausich; Creative Commons Attribution 3.0 Unported license.
Arms
Crinoid arms serve three major functions: respiration, suspension feeding, and locomotion. The large surface area of the arms facilitates gas exchange in respiration. As passive suspension feeders, crinoids also rely on their arms to capture tiny food particles from the water column. Each arm is lined with tube feet—extensions of the water vascular system—covered in a sticky mucus. When a food particle is captured by one of the tube feet, the crinoid uses its tube feet to move the particle moved into an ambulacral groove. From there, the food moves to the mouth. Watch the video below to see a living crinoid suspension feeding.
Watch a feather star feeding. In the close up (17s - 27s), you can see the feather star use its tube feet to catch food particles and transfer those particles to the ambulacral groove, which leads to the mouth. "VERY SPINY FEATHER STAR - 바다나리 류" by Sea School. Source: YouTube.
The final major function of crinoid arms is locomotion in some post-Paleozoic crinoids (watch the videos below to see the crinoids in action). Early crinoids, during the Paleozoic, did not have the capacity to move and, instead, attached themselves to the place where they settled from the water column as larvae. Towards the second half of the Paleozoic, some crinoids may have evolved the capacity for locomotion (see the figure on generic diversity below), potentially crawling with their arms and cirri. More mobile, stalkless crinoids belonging to extant clades (e.g., comatulids), followed in the Late Triassic. Stalkless crinoids can move either by crawling along the ocean bottom or by using their arms to actively swim. When crawling, stalkless crinoids use their arms to pull themselves across the substrate and, interestingly, this behavior has also been observed in some stalked crinoids. As such, the crawling behavior may have served as an intermediary evolutionary step towards active swimming. Today—and presumably for the millions of years since the Late Triassic—many comatulid crinoids swam using alternating strokes of the arms, powered by contraction of their muscles. It has been hypothesized that swimming and crawling behaviors help crinoids avoid predators. For example, many feather stars are nocturnal, hiding from predators during the day, but crawling to a feeding perch at night. Similarly, stalked crinoids can crawl away from a predator or move to find more suitable habitat for suspension feeding (learn more in the paleoecology section below).
Check out the arms and cup of this fossil monobathrid crinoid Actinocrinites gibsoni from the Carboniferous (Mississippian) Edwardsville Formation of Montgomery County, Indiana (PRI 78779). A feeding gastropod, Platyceras (Orthonychia) equilaterum, is associated with this specimen (PRI 78780). Specimen is from the research collection of the Paleontological Research Institution, Ithaca, New York. Length of crinoid is approximately 10 cm. Model by Emily Hauf.
Cup
The crinoids' arms attach at their base to the cup (or calyx), which contains the vital organisms. The cup is encased by plates, providing structure and protection. The topside of the cup, also referred to as the oral side (as opposed to aboral), contains both the mouth and, typically, the anus, which are connected by a U-shaped gut. Around the mouth, which is usually central in the cup, ambulacral grooves carry food from the arms using small cilia (tiny moving hairs) to transport the small food particles captured by filter feeding.
The crown of a camerate crinoid, Eucalyptocrinites caelatus, from the Silurian period of Niagara County, New York (PRI 70772). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Specimen is approximately 5 cm in length and represents the calyx and arms of the crinoid animal.
Columnals
The majority of the stalk (column) is a series of columnals, which begin immediately below the cup. Though the stalk has been lost in many modern comatulid crinoids (at least in their mature, adult forms), columnal components of the stalk are among the most common Paleozoic fossils. These skeletal components, also called columnals, are preserved in such great abundances that they were purportedly used as jewelry in North America, the United Kingdom, and Germany, where they were referred to as "Indian beads," "St. Cuthbert's beads," and "St. Boniface pennies," respectively. When a crinoid is alive, the columnals are connected by soft tissues; however, when the crinoid dies, these tissues decay and leave behind a hole in the center of each columnal. Because a crinoid stalk is made up of potentially dozens of columnals, which break apart after the animal dies, they leave behind a great many fossils. Take a closer look at columnals in the image and 3D models below.

Devonian rock from Upstate New York with an abundance of fossil crinoid columnals. Photo by Jansen Smith.
A piece of crinoidal limestone consisting of hundreds of indvidual crinoid stem segments, from the Mississippian Keokuk Limestone of Monroe County, Indiana (PRI 76734). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 8.5 cm. Model by Emily Hauf.
Fossil specimen of an articulated series of crinoid stem segments from the Pennsylvanian of Tulsa, Oklahoma (PRI 76733). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Specimen is approximately 4.5 cm in length. Model by Emily Hauf.
Holdfast
In the living organism, the stalk is primarily a support structure used to elevate the food gathering portions of the animal into the water column. In early crinoids from the Paleozoic, an attachment region, called a holdfast, was present at the base of the stalk and was used to cement the crinoid to hard substrates.
Fossil specimen of the holdfast of the camerate crinoid Eucalyptocrinus sp. from the Silurian Waldron Shale of Shelby County, Indiana (PRI 76776). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of rock is approximately 9.5 cm.
Starting during the Ordovician, many crinoids evolved holdfast structures for attachment to a greater variety of substrates. In some groups of more modern crinoids (e.g., isocrinids), the holdfast has been replaced by a set of cirri, which can release and regrasp the ocean bottom, allowing the crinoid to move. The cirri can also occur along the stalk in some crinoids and these are also used to grasp and attach to substrate. Because crinoids can still be found alive today, we can study living individuals to learn more about the anatomy and physiology of this group. Watch the video below to learn more about crinoids.
Learn more about crinoids from crinoid researcher Charles Messing in this video entitled, "Sea Star on a Stick: Introducing Crinoids" by Charles Messing. Source: Vimeo.
Crinoid diversity through time
The evolutionary history of crinoids and their diversity can be broken into two general segments, Paleozoic and post-Paleozoic—three distinct Paleozoic crinoid faunas have been discussed in the literature but, for simplicity, will be considered them together here. After first evolving in the Ordovician, crinoids were a prominent member of ocean bottom communities in the Paleozoic. Like many other organisms, crinoids were hard hit by the Permo-Triassic mass extinction. In a severe evolutionary bottleneck, as few as one genus survived the extinction. Post-Paleozoic crinoids eventually regained the ecological diversity of their predecessors but never recovered the variety of morphological forms found from the Paleozoic. To date, more than 6,000 fossil crinoid species have been decribed, and at least 660 modern species are known. Modern crinoid genus-level diversity has nearly matched Paleozoic levels as sea lilies and feather stars continue to evolve. The abundance of modern genera compared to Cenozoic diversity is likely an artefact of sampling because shallow-water feather stars do not live in a habitat that favors preservation and deep-water rocks are rarely preserved where they can be sampled for macrofossils.
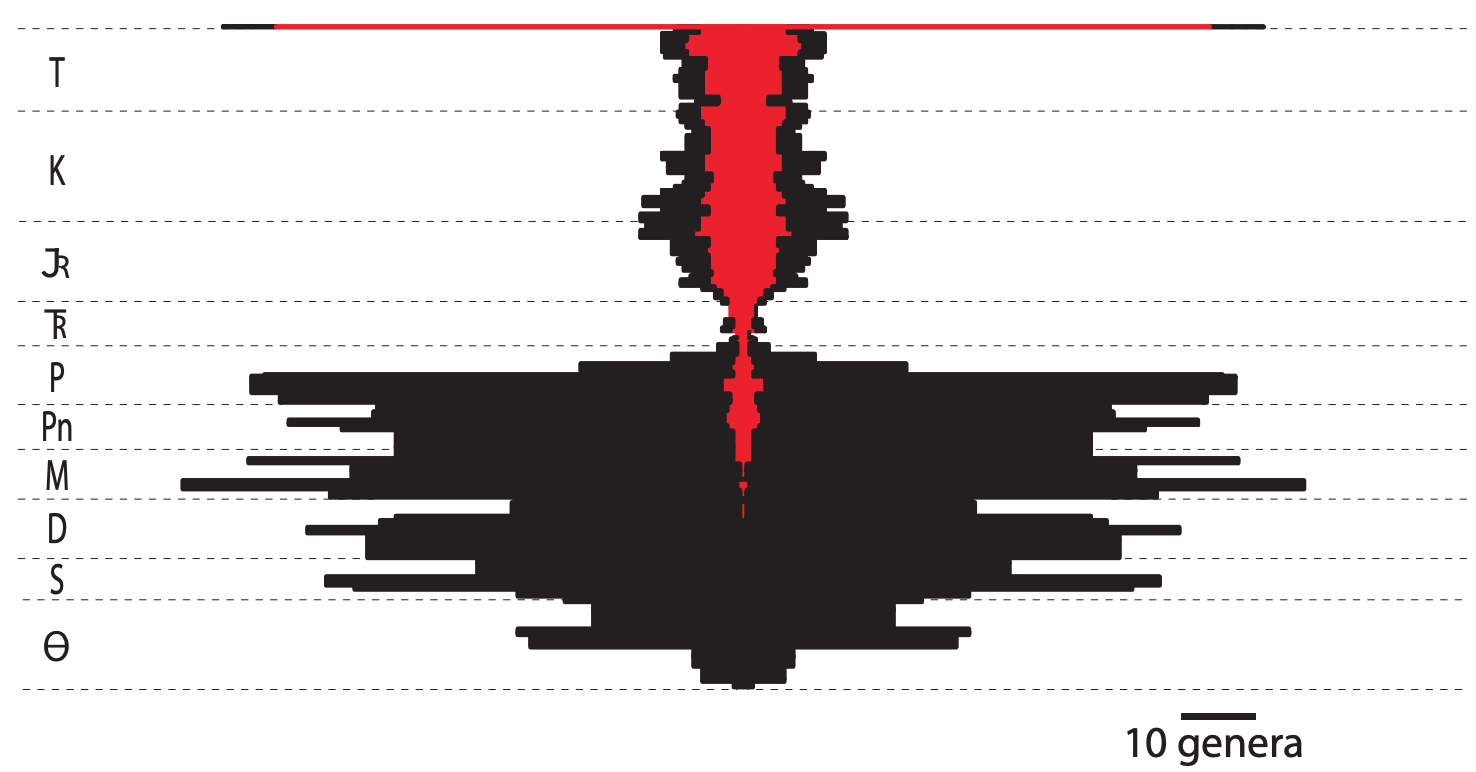
"Generic diversity of crinoids through the Phanerozoic showing the relative proportions of taxa possessing locomotory traits (red) and those lacking such traits (black). Locomotory traits include muscular arm articulations with a well-developed fulcral ridge, fulcrum-bearing cirri distributed along the length of the stalk, absence of a cementing or root-like mode of attachment: for the Paleozoic, this includes only some cladid genera, while for the Mesozoic and Cenozoic, the comatulids, isocrinids, and holocrinids. Generic data from Sepkoski (2002) and Webster (2003)." Figure and caption from Baumiller and Messing (2007) in Paleontologica Electronica; Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license.
Paleozoic crinoids
Crinoids first appeared in the fossil record during the Tremadocian Stage (485.4 – 477.7 million years ago) of the Ordovician and became diverse during the Great Ordovician Biodiversity Event—a time when many clades of early life diversified. In the Early Ordovician, most crinoids were obligate hard-substrate taxa with relatively primitive holdfasts (for examples, see the 3D models below). They were commonly a dominant component of nearshore, hardground ecosystems. During this early Paleozoic time, crinoids possessed relatively sparse arrays of arms, each lined with a large ambulacral groove; however, these features changed quickly as the group diversified and specialized. By the Middle Ordovician, crinoids evolved a broader set of holdfast types, allowing them to move beyond strictly hardground substrates. Crinoids were hard hit during the end-Ordovician mass extinction event, with groups like the Diplobathrida, Disparida, and Hybocrinida losing more than 75% of their genera. This extinction, and the subsequent recovery, marks the transition from the Early to the Middle Paleozoic crinoind evolutionary fauna.
The fossil disparid Daedalocrinus sp. from the Ordovician period (PRI 49826). Disparids such as this one were common in deeper, lower energy environments. This specimen is on public display at the Museum of the Earth, Ithaca, New York. Length of specimen is approximately 7.5 cm.
Fossil specimens of the cladid crinoids Stellarocrinus bilineatus (PRI 49827) and Brabeocrinus christinae (PRI 76675) from the Pennsylvanian Bond Fm. of Livingston County, Illinois. Specimen is on display at the Museum of the Earth, Ithaca, New York. Longest dimension of rock is approximately 8.5 cm.
Crinoids diversified in the Silurian and Devonian until reaching their maximum fossil diversity during the Early Carboniferous—this time is also called the Mississippian Period. During the middle of the Paleozoic, the first muscular arm articulations evolved in the cladid group, which eventually gave rise to all post-Paleozoic diversity. The evolution of muscles is notable, as these muscles contribute to crinoid flexibility and locomotion, with potential for rapid movement. In fact, the crinoid capacity to move likely evolved during the Devonian, potentially in association with the innovation of muscles.

A comatulid crinoid, Tropiometra carinata, demonstrating the use of cirri for substrate attachment. This mode of attachment is common in modern crinoids and likely began to evolve by at least the Mississippian. Photo by Seascapeza; Creative Commone Attribution-Share Alike Unported 3.0 license.
The Mississippian is often referred to as the “Age of Crinoids” because of the biodiversity and shear abundance of crinoids—it was during this time that the Late Paleozoic crinoid evolutionary fauna arose. By this time, most crinoids had evolved to use rooted or cirri attachments rather than the cemented holdfasts common in early Paleozoic crinoids. Though the vast majority of these crinoid species still were not capable of movement, evolution away from cemented holdfasts enabled them to live in a wider variety of environments, regardless of the substrate. As the Paleozoic Era came to a close, Crinoid diversity had already begun to decline before the decimation of the end-Permian mass extinction. Only one lineage of crinoids, the Articulata, survived the mass extinction and some evidence suggests only a single genus persisted.
Post-Paleozoic crinoids
Following the Permo-Triassic mass extinction, crinoids underwent a major evolutionary radiation. With this radiation, many crinoid species evolved the capacity for movement and, by the end of the Middle Triassic, mobile crinoids were in the majority, compared to immobile forms. Although there was species turnover—similar rates of extinction and origination of species—crinoids passed through the end-Triassic mass extinction with relatively little loss of diversity.

Free-living Uintacrinus socialis specimens from the Cretaceous of Kansas. These stalkless crinoids likely lived in floating colonies, making them somewhat unique in their life habit compared to other crinoids. Photograph by Jonathan R. Hendricks of specimens on display at the Denver Museum of Nature and Science.
Crinoid diversity during the Mesozoic reached its peak towards the end of the Jurassic. Despite the dominance of mobile crinoids in the Triassic, sessile (i.e., non-moving) crinoids diversified rapidly throughout the Jurassic. Though many of the sessile genera survived into the earliest Cretaceous, most of them went extinct before the Middle Cretaceous. From that point onward, mobile crinoids have dominated the crinoid fauna.

An array of Jurassic Seirocrinus and Pentacrinites (members of the Pentacrinitidae famliy) attached to a piece of driftwood. This specimen is on display at the Urwelt-Museum Hauff Holzmaden. Image by Ghedoghedo; Creative Commons Attribution-Share Alike 3.0 Unported license.
Mesozoic crinoids never recovered the morphological diversity that they exhibited during the Paleozoic but equaled, if not exceeded, the ecological diversity of their predecessors. As discussed by Michael Foote (1999), the restriction in morphological diversity may be due to genetic constraint. That is, the crinoids that survived the Permo-Triassic extinction—potentially a single genus—did not have the genetic tool kit required to “build” the types of features found in Paleozoic crinoids. This effect, called a bottleneck, limited the variety of forms that were possible for Mesozoic crinoids.
Starting in the Mesozoic and continuing into the Cenozoic, predation on crinoids contributed substantially to the dominance of mobile genera. During the Mesozoic, escalating ecological processes led to the Mesozoic Marine Revolution, wherein predators became more powerful and more efficient. Consequently, the ability to escape predators would have been a significant advantage for mobile crinoids. Mobile crinoids remain the most dominant forms today, comprising as much as 85% of the fauna. Of the stalked crinoids that remain, none live naturally in depths less than 150 meters. The presence of more active predators in the shallower ocean has relegated these once dominant forms to deeper ocean habitats.

Examples of living crinoids, including an isocrinid (top left) and two comatulida (bottom left; right). Top left image from NOAA (public domain). Bottom left image from NOAA (public domain). Right image by Alexander Vasenin; Creative Commons Attribution-Share Alike 3.0 Unported license.
Today, crinoids have regained a high level of generic diversity and are represented by more than 660 living species. Though the Cenozoic record of crinoids is depauperate, more than 6,000 fossil species have been described since the Ordovician. The numerous genera of Recent crinoids compared to fossil crinoids of the Cenozoic likely reflects preservational bias, particularly against mobile taxa like feather stars that live in habitats with low preservational potential. Additionally, fossils commonly undergo taphonomic processes, becoming compressed, fragmented, and otherwise challenging to interpret and identify. With continued study, Cenozoic crinoid diversity may increase.
Crinoid (paleo)ecology
All crinoids are suspension feeders, meaning they extract their food from water as it flows past them. Unlike some other suspension feeders that actively pump water past their own feeding apparatuses (e.g., some sponges, bivalves), crinoids are considered passive suspension feeders because they only capture food particles and do not manipulate the flow of water as it passes them. Early crinoids, such as those dominant during much of the Paleozoic, were often cemented to a single location and unable to move. As such, it was critical for these stationary animals to be situated in a location where water currents facilitated suspension feeding. Although the adult organisms were stationary, the crinoid larvae were, and still are, plankontic. Considering living crinoids and other organisms with similar life habits, it is likely that the crinoid larvae were able to detect suitable locations before settling out of the water column and growing into adults. To learn more about how crinoids are studied, and particularly how studies of living crinoids are used to understand fossil crinoids, watch the video below.
Learn more about crinoids, and how they are studied, in this video, "Living Fossils - Full Episode" by ChangingSeasTV. Source: YouTube.
Water currents in the ocean are variable, which means the food supply to stationary crinoids was also variable. To cope with this variability, Paleozoic crinoids evolved slightly different body plans when living in areas with weaker (e.g., deeper water) or stronger (e.g., shallower water) currents. As discussed by Holterhoff (1997), in shallower environments, where water currents were commonly strong, crinoids were more likely to have a large calyx and a dense array of arms and pinnules. To the contrary, fossil crinoids from deeper environments, where water currents were weaker, had a smaller calyx and fewer arms and pinnules. As crinoids evolved the ability to move, particularly toward the end of the Paleozoic, this pattern likely broke down, as individuals could move themselves to an optimal water current for feeding.
During the Mesozoic, individuals in one family, Pentacrinitidae, utilized a particularly novel approach to life. Pentacrinitids, which are an extinct relative of the living isocrinids and potentially an evolutionary descendent of the earliest free-living isocrinids, attached themselves to driftwood and moved with it as it floated around the ocean. Interestingly, a group of barnacles employs a similar strategy today: they attach to whales and and various types of floating debris and suspension feed while moving through the water. With this strategy, the floating pentacrinitids—and some barnacles, today—could access a broader array of food resources than individuals attached to the ocean bottom. Despite this innovation, pentacrinitids went extinct during the Eocene Epoch of the Cenozoic.

A reconstruction of Pentacrinites attached to a piece of driftwood. Image by NobuTamura; Creative Commons Attribution-Share Alike 3.0 Unported license.
In today’s oceans, the majority of crinoids are capable of moving themselves. Some species of comatulids (i.e., feather stars) are even capable of swimming for short periods. Those that cannot swim, including some feather stars and the isocrinids, move instead by crawling along the seafloor. By being mobile, these crinoids can position themselves for more efficient filter feeding and, critically, mobility allows the crinoids to escape some of their predators, like sea urchins and fish.
A swimming comatulid crinoid. "Rare Moment Feather Star is Caught Swimming" by Caters Clips. Source: YouTube.
Watch as a stalked isocrinid, Neocrinus decorus, crawls away from a predatory sea star (off camera). "Crawling Crinoid" posted by gamecraziness2. Source: YouTube. Video captured by Tomasz Baumiller.
Predation on crinoids
Predation has played an important role in the diversification and distribution of crinoids in space and time. Early on, in the Ordovician and Silurian, there is very little evidence of predation on crinoids; however, from the Devonian onward, evidence of predation becomes abundant. As discussed by Tomasz Baumiller (2008), the evidence of predation can take several forms, including damaged cups and arms, evidence of arm or pinnule regeneration, and crinoid remains preserved in fossilized fish scat. Whereas the capacity to swim and crawl is likely an effective defensive strategy against crawling sea stars and urchins, it is less effective against more agile swimmers like fish. Crinoids have developed an additional defensive strategy, autotomy, that can be deployed against fish, sea stars, and sea urchins alike.
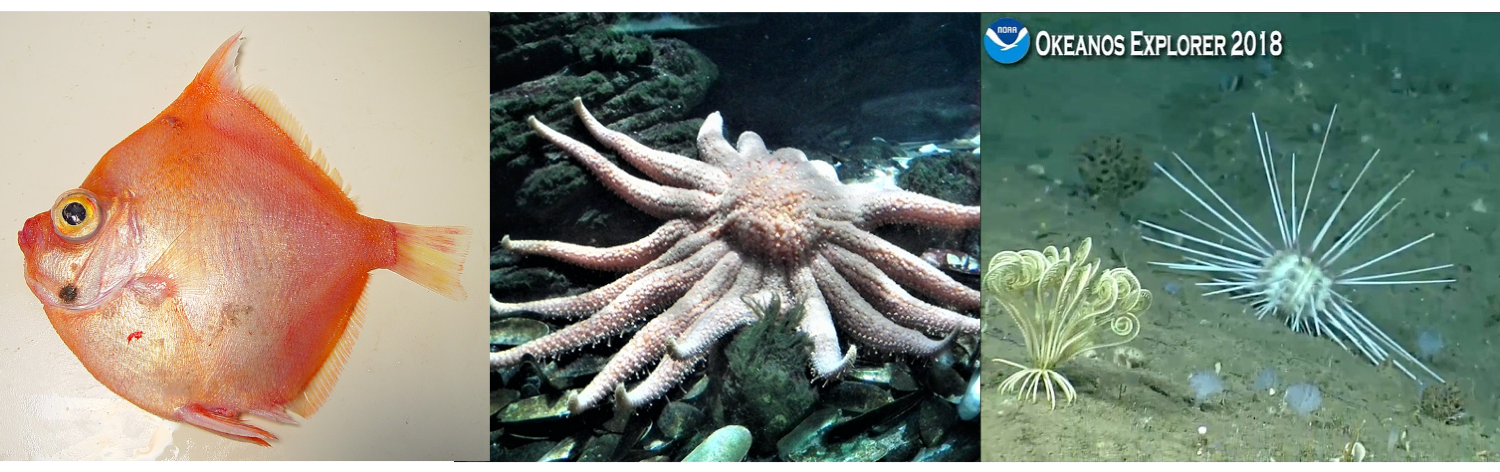
A selection of crinoid predators, including boarfish (Antigonia capros; left), sea stars (Pycnopodia helianthoides; middle), and sea urchins (Calocidaris micans; right). Left image by NOAA/NMFS/SEFSC Pascagoula Laboratory; Creative Commons Attribution 2.0 Generic license. Middle image by MOs810; Creative Commons Attribution-Share Alike 4.0 International license. Right image by NOAA Okeanos Explorer (public domain).
Autotomy is the intentional loss of an appendage and is a defensive strategy that has evolved independently in different branches of the tree of life. For example, some lizards will lose their tails when pursued or captured by a predator. If they are being pursued, the tail often distracts the predator, allowing the lizard to escape. Likewise, losing the tail can be an effective means for getting free from a predator’s grasp. Crinoids do not have tails to lose, but they will often drop an arm, or even their stalk, in order to avoid fatal predation.
Watch as a sea urchin consumes a feather star on the sea floor. Not even autotomy could save the feather star this time. "Sea urchin consumes a feather star crinoid" by UM News Service. Source: YouTube.
Much like the mythical hydra regrowing its heads, when a crinoid loses an arm, it grows back either one or two fully functional arms in its place—many crinoids actually do this in order to increase their number of arms and, thereby, increase their capacity to suspension feed. In addition to observing modern crinoids utilizing this anti-predatory behavior, evidence of arm loss and regeneration can be found in the fossil record, including from the Paleozoic. The proportion of individuals in a community with evidence of autotomy can be highly variable, ranging from 0–100%, but modern and fossil frequencies typically range from 15–30%. Because of their autotomy behavior, predation is not fatal for crinoids in many cases.
Crinoid parasites and commensalists
In addition to antagonistic predator-prey interactions, fossil and modern crinoids interact closely with a variety of other organisms. In the Ordovician through Permian fossil record, a group of snails called platyceratids are often found attached to crinoids (see if you can find it in the 3D model below). Initially, paleontologists in the 1800s thought the crinoids were eating the platyceratid snails because the snails are often found on the top side of the cup, close to the crinoid’s mouth.
Fossil specimen of the monobathrid crinoid Agaricocrinus americanus from the Mississippian Edwardsville Formation of Montgomery County, Indiana (PRI 70601). Also present is a small, parasitic gastropod called Palaeocapulus equilateralis. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Length of crinoid is approximtely 10 cm.
After examination of additional specimens, this hypothesis was rejected in favor of a new explanation: the snails were actually eating the effluent released by the crinoid (i.e., coprophagy). Given the common position of the snails near the anal opening of the crinoids, which is coincidentally located very near the mouth, this explanation portrays the relationship as a commensalism—an interaction between species where one species benefits and the other species neither benefits nor suffers. Continued study of fossil crinoid specimens, particularly studies examining cross-sections from the region where the snails attached to the crinoids, has revealed the presence of small drill holes. These holes suggest that the snails may have bored into the crinoid to consume nutrients straight out of the crinoid gut, and potentially the reproductive organs. That is, the snails may have been parasites. There are several species of platyceratid snail, and they are found on a variety of crinoids, leaving open the possibility for multiple types of interactions. It is clear that the snails benefitted from the interaction by gaining food; however, it remains unclear whether the interaction was always negative from the perspective of the crinoid, or merely a neutral one in some cases.
Modern crinoids, especially comatulids, are also regularly observed with other organisms—including shrimp, lobsters, worms, and fish—living on and amongst their arms. The relationships between the crinoids and these organisms is somewhat variable, ranging from parasitic (e.g., interactions with worms) to mutualisms where both organisms benefit (e.g., interactions with shrimp, lobsters, and fish). The crinoids serve as a host and, indirectly through their larger size, protect the smaller organisms from their predators. The smaller organisms also benefit by feeding on food trapped amongst the crinoids’ arms and pinnules. Through this feeding, some of the smaller “hitch hikers” clean the crinoid, which may benefit the crinoids. Watch the video below to learn more about these relationships and to see the organisms in action.
Learn more about crinoid ecology in this video on "Feather Stars and Their Animal Invaders" by Nat Geo WILD. Source: YouTube.
References and further reading:
Ausich, W. I. 1980. A model for niche differentiation in Lower Mississippian crinoid communities. Journal of Paleontology, 54: 273-288.
Ausich, W. I. 1998. Early phylogeny and subclass division of the Crinoidea (Phylum Echinodermata). Journal of Paleontology, 72: 499-510.
Baumiller, T. K. 2008. Crinoid ecological morphology. Annu. Rev. Earth Planet. Sci., 36: 221-249.
Baumiller, T. K., and F. J. Gahn. 2018. The nature of the platyceratid–crinoid association as revealed by cross-sectional data from the Carboniferous of Alabama (USA). Swiss Journal of Palaeontology, 137: 177-187.
Cole, S. R., D. F. Wright, and W. I. Ausich. 2019. Phylogenetic community paleoecology of one of the earliest complex crinoid faunas (Brechin Lagerstätte, Ordovician). Palaeogeography, Palaeoclimatology, Palaeoecology, 521: 82–98.
Foote, M. 1999. Morphological diversity in the evolutionary radiation of Paleozoic and post-Paleozoic crinoids. Paleobiology, 25: 1-115.
Gorzelak, P., M. A. Salamon, D. Trzęsiok, R. Lach, and T. K. Baumiller. 2016. Diversity dynamics of post‐Palaeozoic crinoids–in quest of the factors affecting crinoid macroevolution. Lethaia, 49: 231-244.
Hess, H., W. I. Ausich, C. E. Brett, and M. J. Simms. 2002. Fossil crinoids. Cambridge University Press, New York, New York, 300 pp.
Holterhoff, P. F. (1997). Paleocommunity and evolutionary ecology of Paleozoic crinoids. The Paleontological Society Papers, 3: 69-106.
Wright, D. F., W. I. Ausich, S. R. Cole, M. E. Peter, and E. C. Rhenberg. 2017. Phylogenetic taxonomy and classification of the Crinoidea (Echinodermata). Journal of Paleontology, 91: 829-846.
Usage
Unless otherwise indicated, the written and visual content on this page is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 International License. This page was written by Jansen A. Smith. See captions of individual images for attributions. See original source material for licenses associated with video and/or 3D model content.




