Introduction
The sporophytes of most vascular plants branch within the normal course of their growth and development. Branching occurs when one axis (root or stem) divides, or when a smaller axis develops on a larger, more dominant axis. Branching of axes can occur apically (at the apex or tip of an axis) or laterally (on the side of an axis). Apical branching is the more ancient type of branching in both stems and roots; it is also structurally similar in stems and roots. True lateral branching developed in the euphyllophytes (the group including horsetails, ferns, and seed plants, as well as their extinct ancestors and relatives) and takes places via different processes in shoot and root. In shoots, lateral branches develop from buds on the surface of the stem; lateral roots originate within the root from which they branch.
The first sections of this page will cover branching in shoots. Topics include apical branching, lateral branching, and adventitious branching. Next, monopodial and sympodial growth in laterally branching plants will be explained. Finally, root branching is briefly discussed at the end of this page.
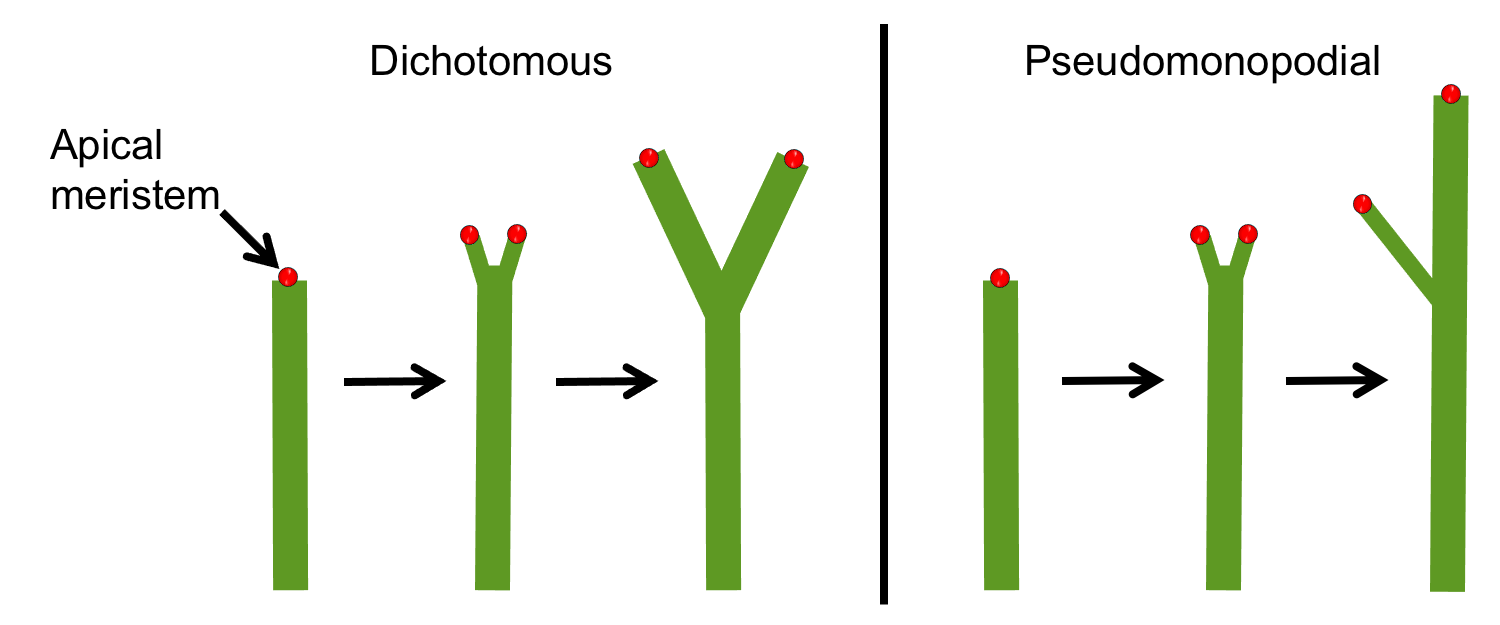
Apical branching (dichotomous branching)
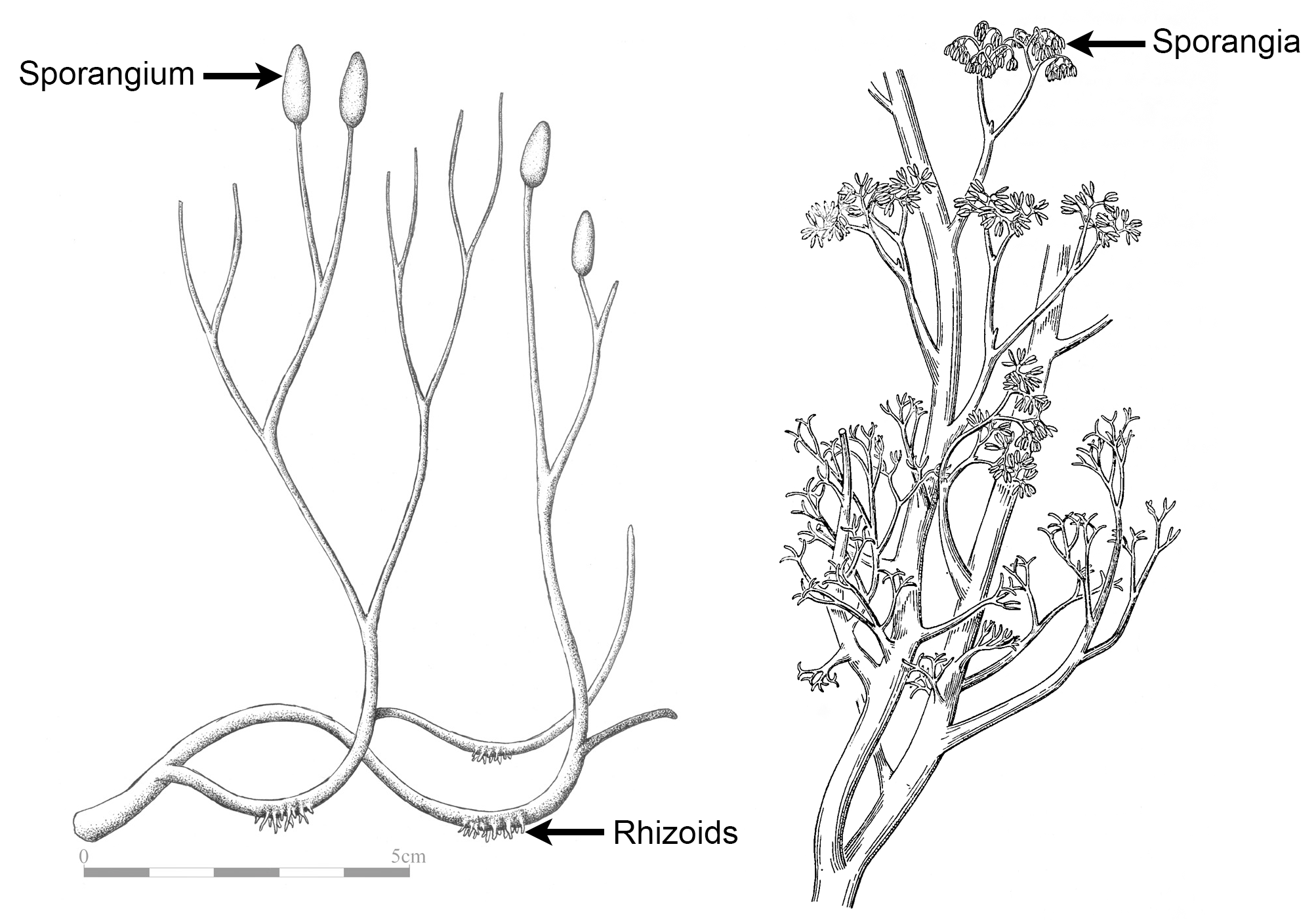
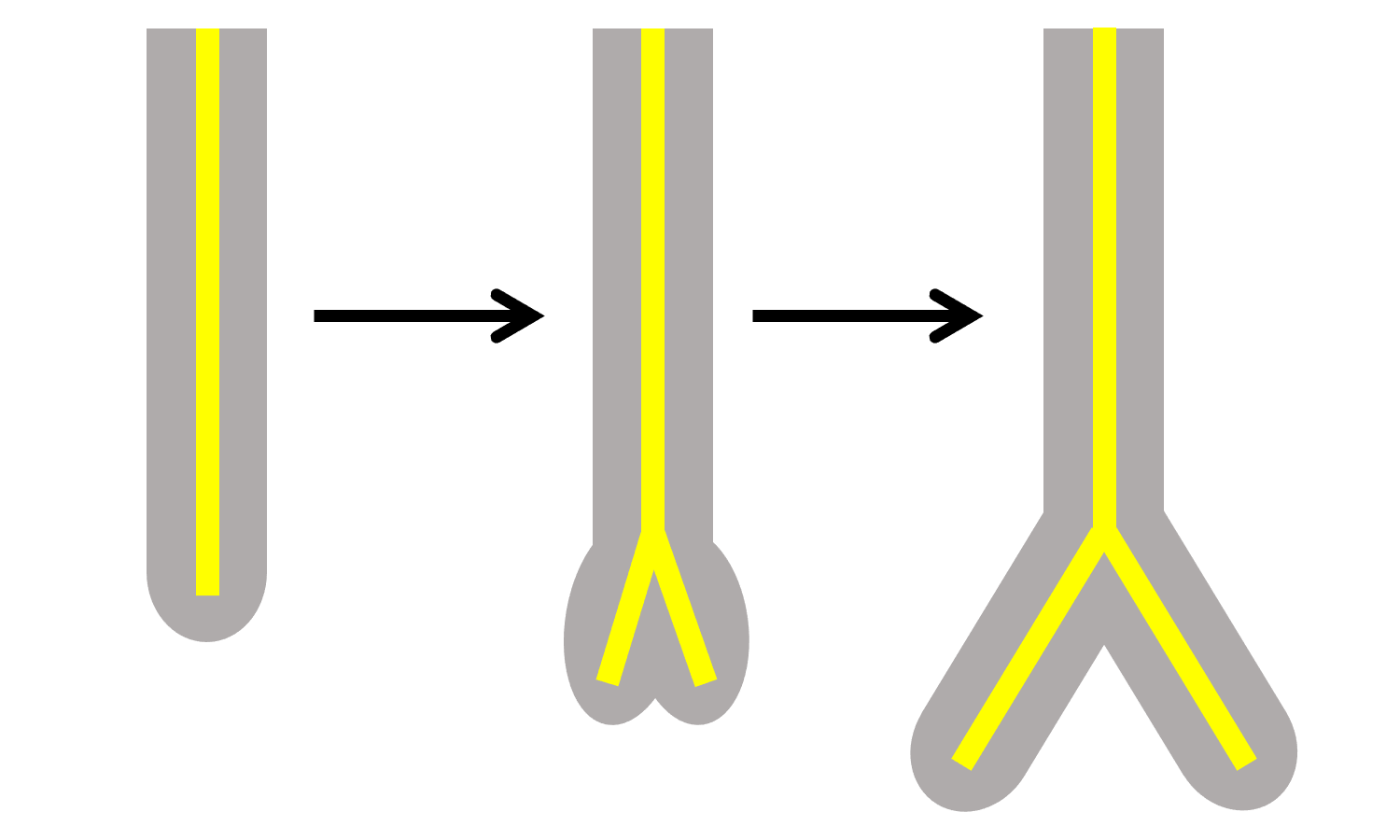
Apical branching is a type of branching in which the shoot apex divides, usually bifurcating to produce two branches. In the simplest type of apical branching, the apex divides to produce two equal branches. This type of apical branching is often simply called dichotomous (Greek dicho- = in two), but is sometimes called isotomous (Greek isos = equal). Dichotomous branching was the earliest type of branching and is seen in some ancient plant sporophytes, like the Silurian to Devonian plant Cooksonia (typically considered an early vascular plant) and the Devonian plant Aglaophyton (a protracheophyte, a branching plant lacking true vascular tissue, shown below). Dichotomous branching is also seen in some modern plants like the firmosses (Huperzia, an example is shown below) and whisk ferns (Psilotum).
Sometimes, apical branching produces branches of unequal size. This type of branching is known as anisotomous (Greek anisos = unequal). If apical branching is extremely unequal, it may be called pseudomonopodial (Greek pseudēs = fake/false). A plant with pseudomonopodial branching or pseudomonopodial growth mimics the form of a plant with monopodial growth (discussed below). In other words, pseudomonopodial branching results in a plant that looks like it has a dominant ("main") stem and lateral (side) branches, even though branching is really apical. Unequal apical branching occurs in lycophytes and some extinct groups, like trimerophytes (the stem group to euphyllophytes, the group that includes ferns, horsetails, and seed plants).
Terminology note: "Dichotomous branching" is often used as a synonym for apical branching, encompassing both isotomous (equal) and anisotomous (unequal) types. In this case, a modifier (like "isotomous" or "equal") may be used to indicate the relative size of the branches.

Lateral branching
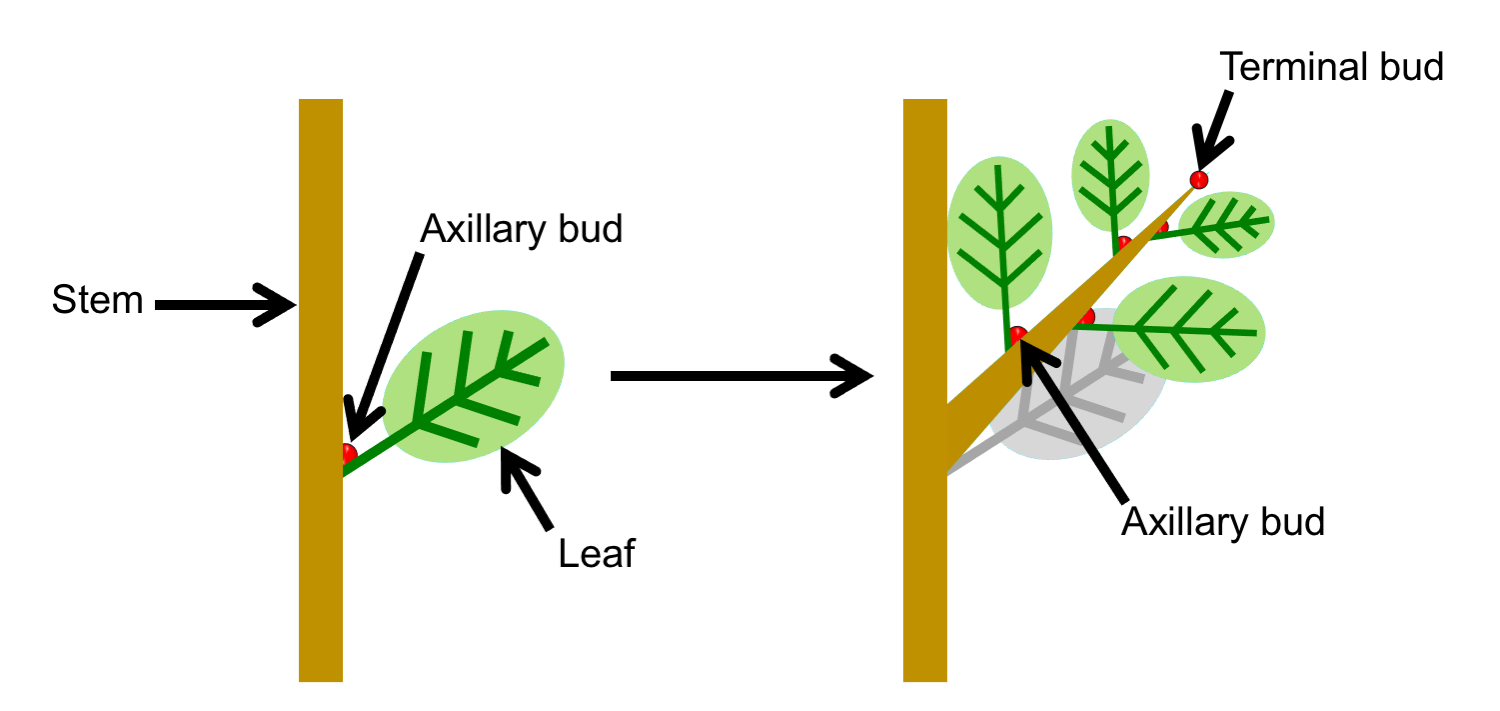
In plants with lateral branching, a main stem grows from an apical bud or terminal bud (a region of the shoot tip that includes the apical meristem), and lateral (side) branches are produced from lateral buds, each with its own apical meristem. Thus, branching is not caused by division of the shoot apex.
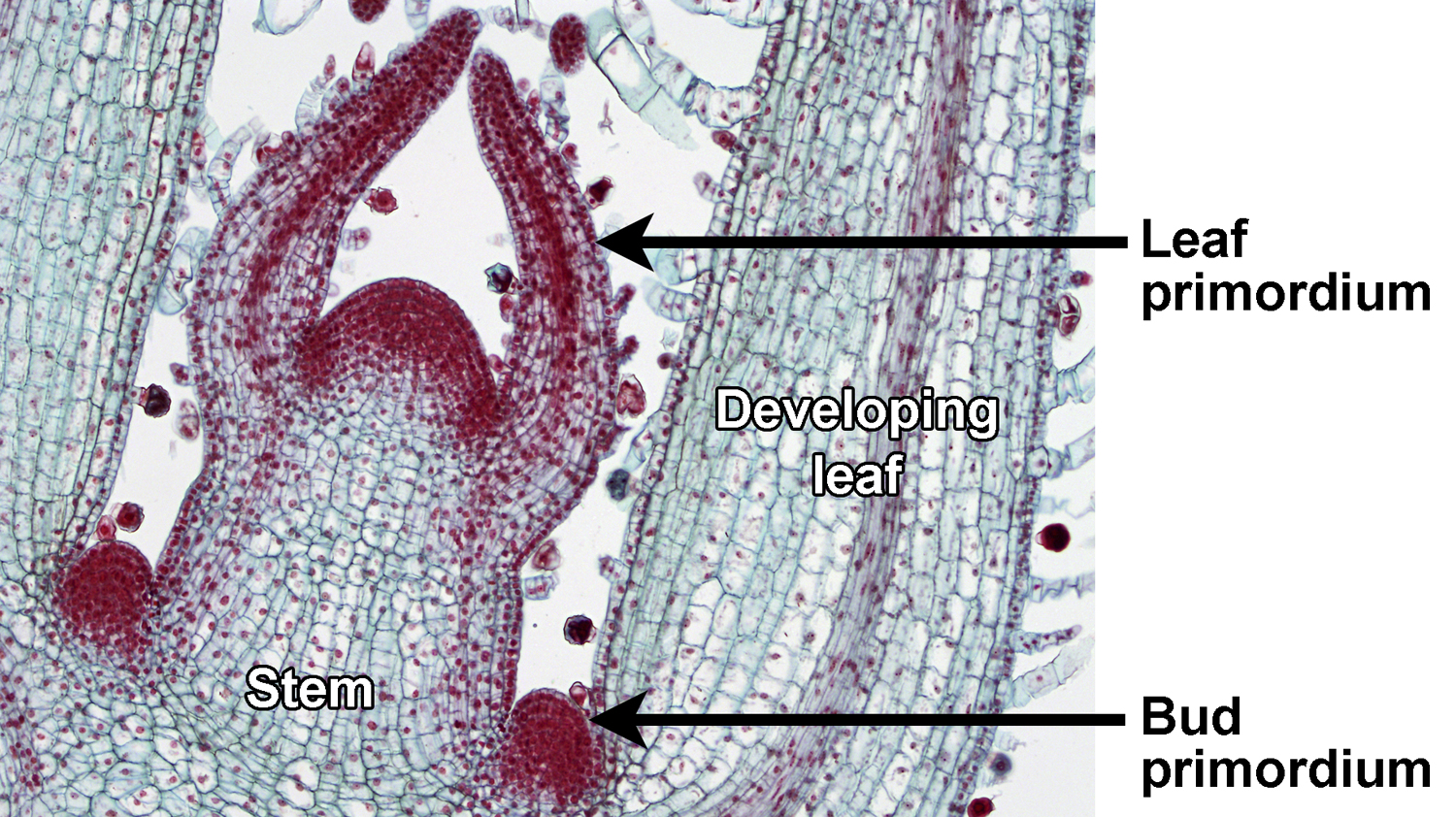
Axillary branching
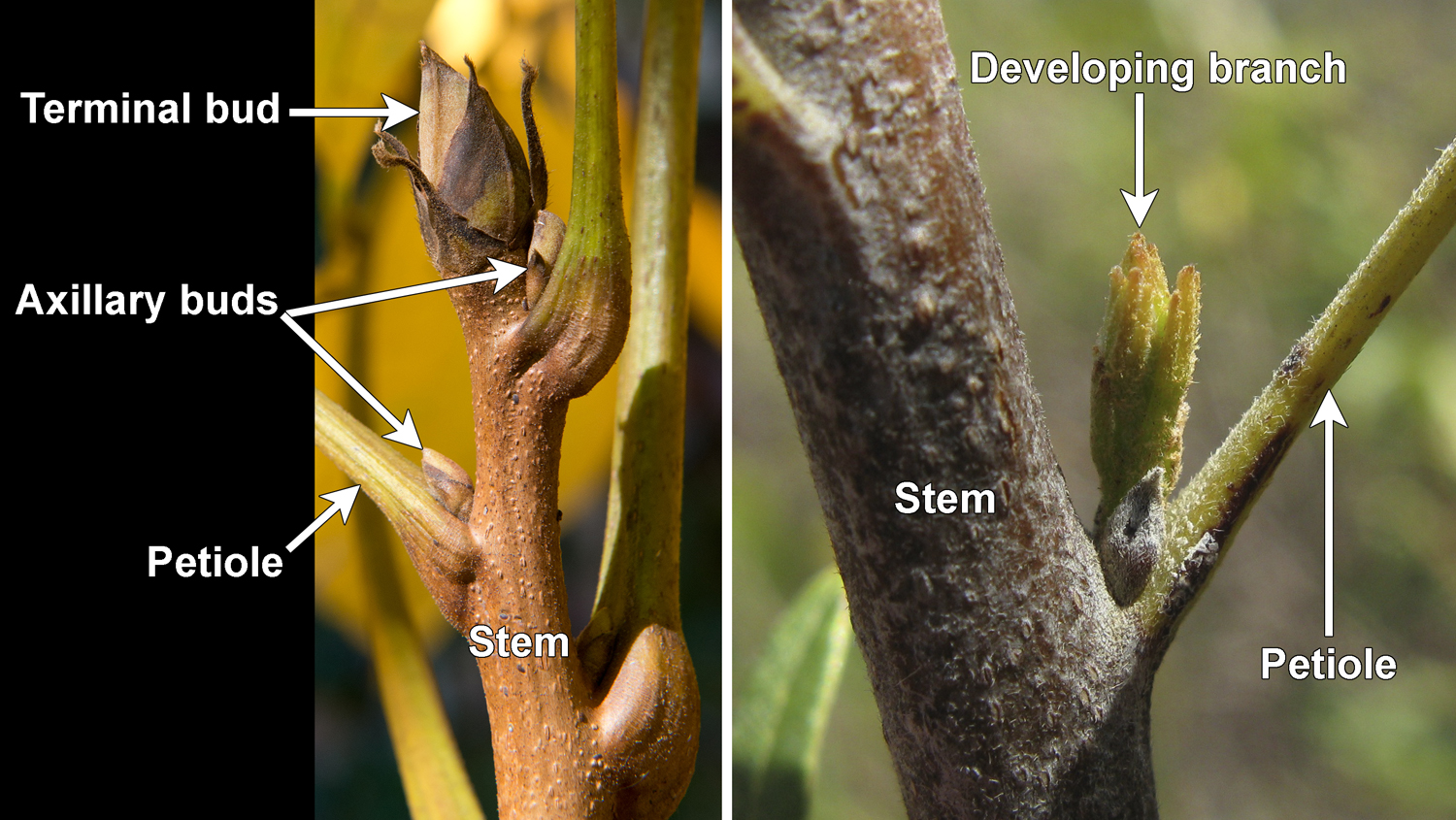
In axillary branching, lateral branches grow from axillary buds, or buds the develop in the leaf axils. An axil is the angle formed between the the upper (adaxial) side of the leaf and the stem to which it is attached; the word axil comes from the Latin word axilla, meaning "armpit" (just remember: the axil is the armpit of the leaf!). Axillary buds—also called lateral buds—are just immature branches. An axillary bud may elongate to form a vegetative (sterile) branch, but it may also develop to form a fertile branch. A fertile branch is a reproductive structure like a cone or a flower. Axillary branching is found in many seed plants, including ginkgoes (Ginkgo), conifers, and angiosperms (flowering plants); axillary branching is absent in cycads.
Terminology note: Often, the term "axillary bud" is used when leaves are present, as "axillary" describes the position of the bud in the upper angle between leaf and stem. The term "lateral" may be preferred when leaves are shed (lost), because the position of the bud is no longer obviously axillary (without a leaf, there is no axil).

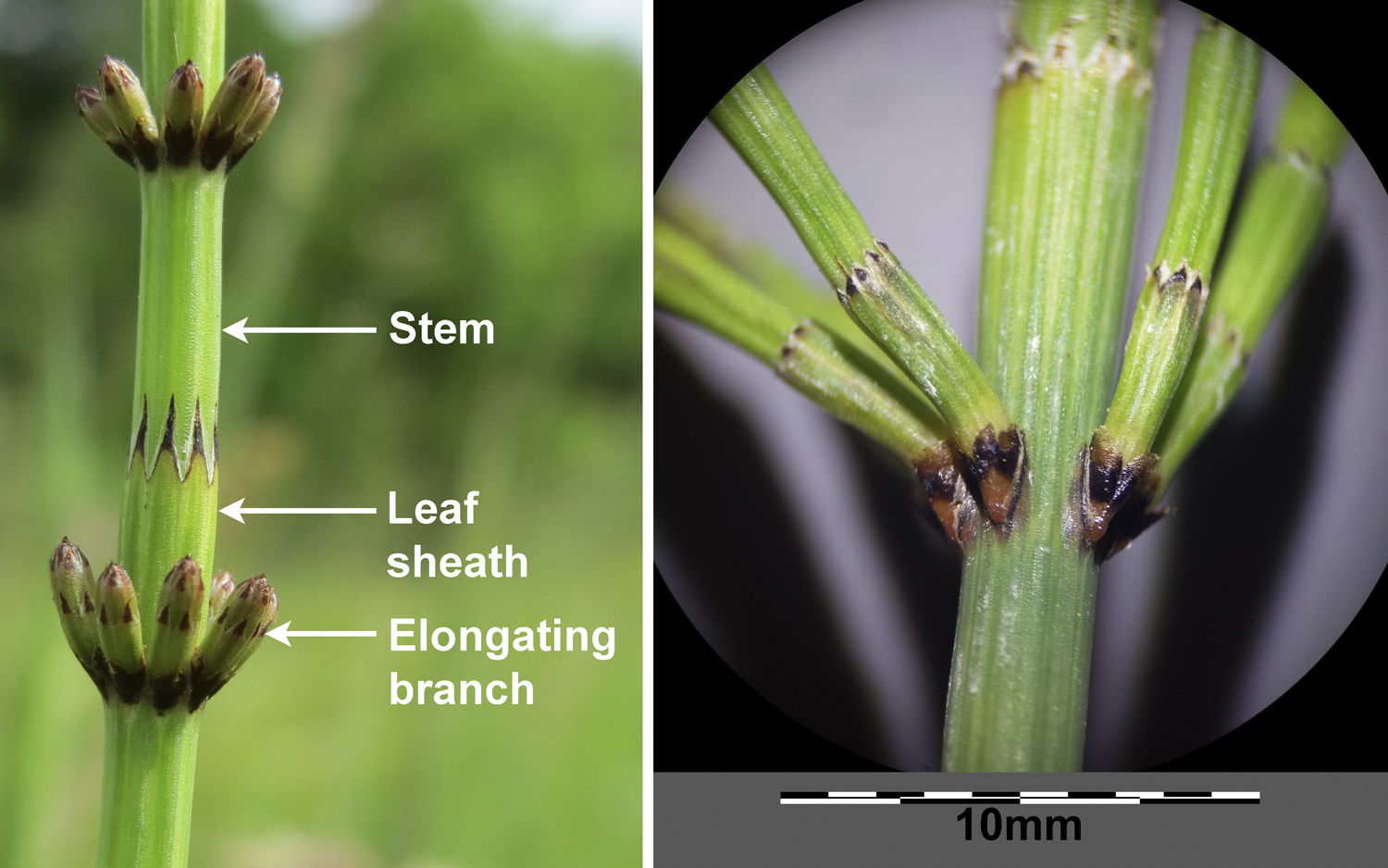
Non-axillary lateral branching in pteridophytes
While branching in seed plants is typically axillary, lateral branches can also arise from non-axillary positions on a stem. In ferns, lateral branches can arise from a variety of positions on the stem. In horsetails (Equisetum), lateral branches originate from positions between the leaves. Because the tiny leaves in horsetails are fused into a sheath around the stem, the branches break through the leaf sheath during growth.
Adventitious branching
Adventitious branching is branching that does not occur via the normal process of branching for a given plant. In general terms, adventitious structures are structures that develop in unexpected places. Note that "unexpected" means unexpected from the perspective of the organization of the plant embryo and standard plant body plan. Adventitious structures are rather common in plants, and, in fact, can be an essential part of the development of some types of plants. For example, adventitious roots (stem-borne roots) replace the primary root (the root the develops from the embryonic root) in many pteridophytes and monocots as the plants transition from embryo to mature forms.
For an apically branching plant, adventitious branches are branches that do not develop by division of the shoot apex, but arise elsewhere. For plants with axillary branching, adventitious branches grow from buds that develop in a region away from the leaf axil (or former leaf axil, if the leaf has been shed). Cycads, which typically branch apically, can form adventitious lateral branches. In this case, suckers (also called bulbils, offsets, or pups) may form on the sides of stems, from tissue in the leaf bases.

Monopodial & sympodial growth
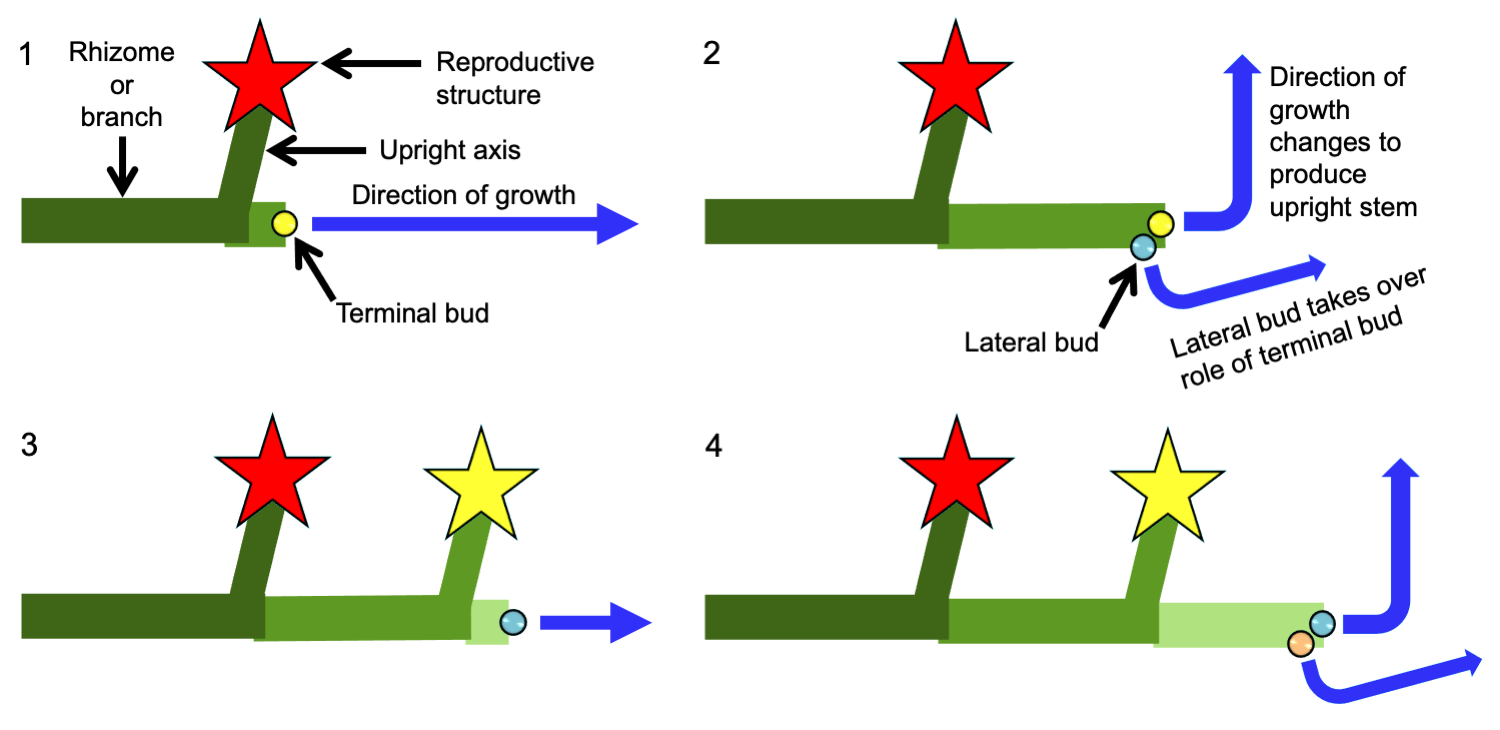
Plants with lateral branching may exhibit either monopodial or sympodial growth. In monopodial growth (Greek, monos = one + Latin, podium = foot), a stem elongates via new growth produced by a single terminal bud. In sympodial growth (Greek, sym = together + Latin, podium = foot), a stem elongates via new growth produced by a successive series of lateral buds that take on the role of the terminal bud. Thus, in sympodial growth, a stem is made up of growth produced by multiple, separate buds in a sequence.
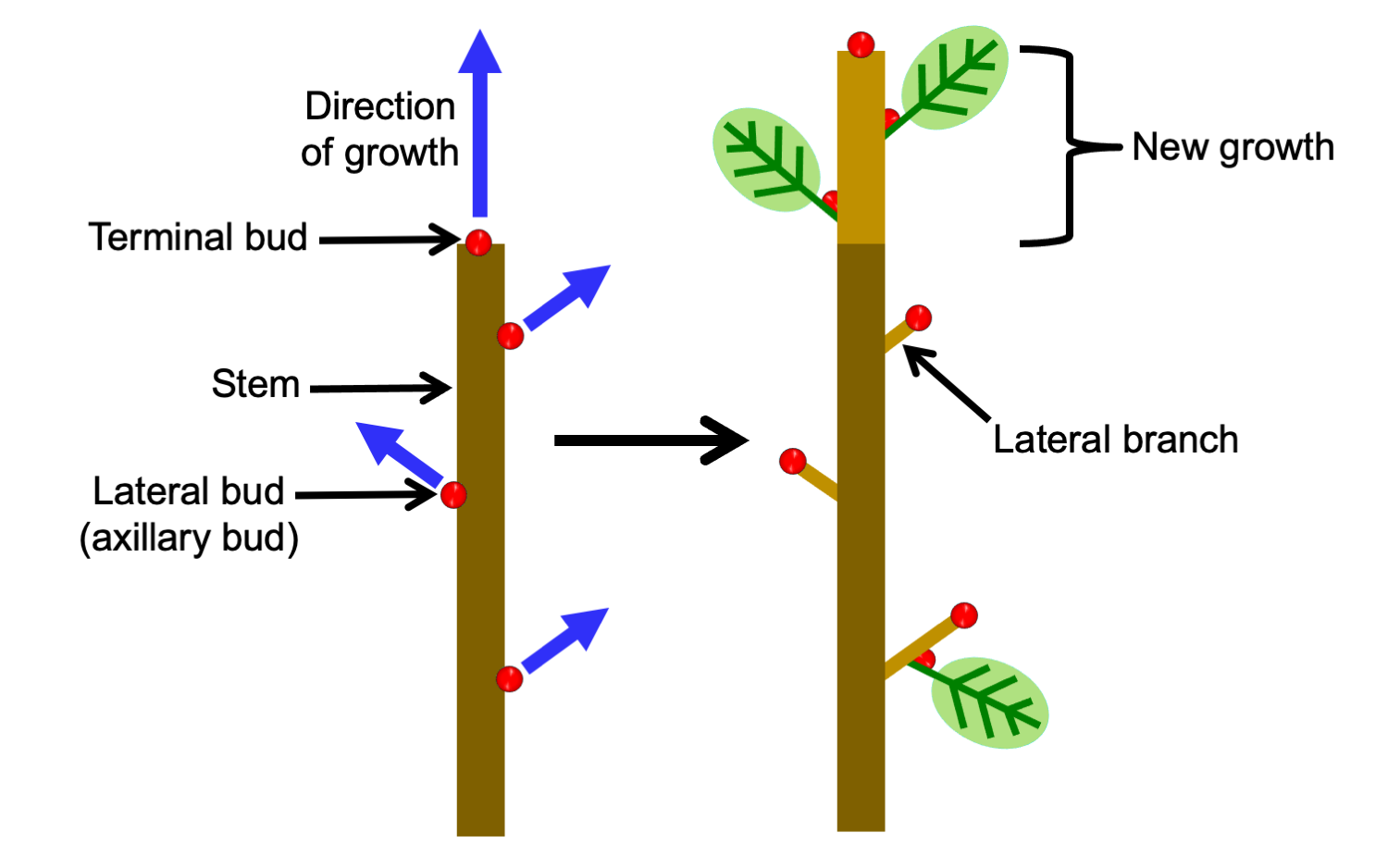
Monopodial growth
In monopodial growth, a stem elongates from a single, terminal shoot apex. Lateral branches develop from lateral or axillary buds.
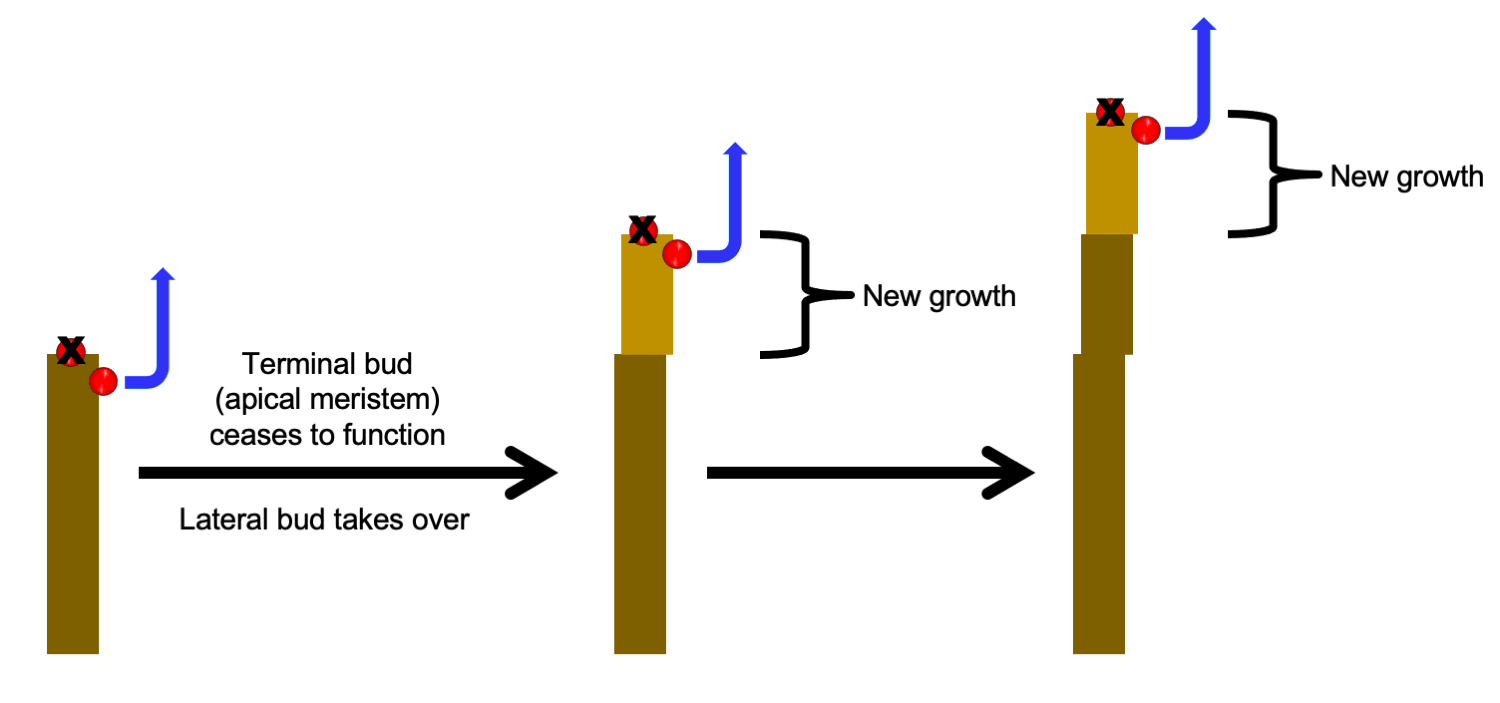
Sympodial growth
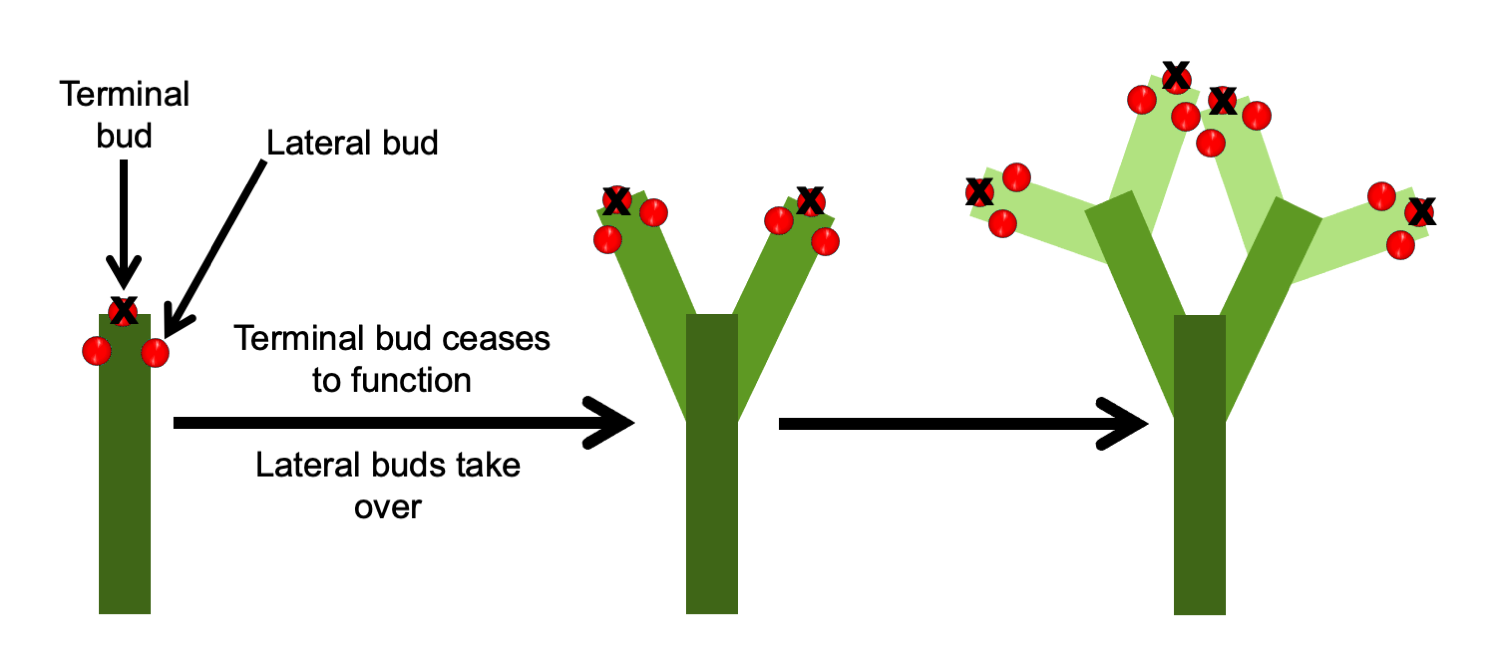
In sympodial growth, a stem is made up of growth from more than one bud. Successive lateral buds take over the role of the shoot apex (terminal bud), adding to the growth of the stem sequentially.
Terminology note: A lateral bud that takes over the role of a terminal bud to produce what appears to be a single continuous stem is sometimes called a false terminal bud or pseudoterminal bud (Greek, pseudēs = false). In the diagrams on this page, a bud is labelled a terminal bud when it serves the role of a terminal bud, regardless of its original developmental position. The distinction between true and false terminal buds can be important in woody plant identification.


Sympodial growth of angiosperm inflorescences
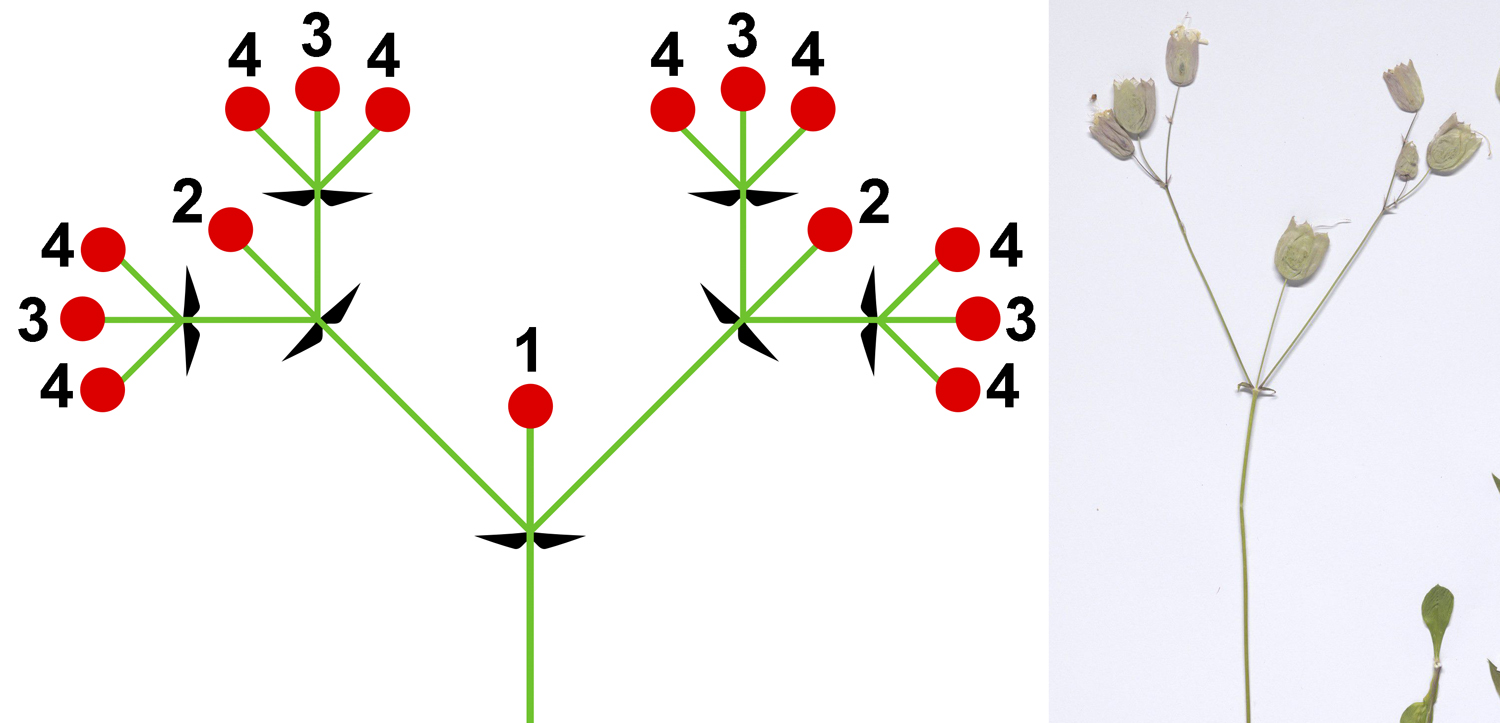
Sympodial growth is sometimes found in angiosperm inflorescences (groups of flowers). If sympodial growth of the inflorescence axis (the stem bearing the group of flowers) continues by one bud at a time in succession, the inflorescence is a monochasium. An example of a monochasium is the scorpioid cyme shown below. Unlike the examples of the rhizome and the plagiotropic branch illustrated above, the positions of the lateral buds that take over the role of the terminal bud in each growth interval alternate in a scorpioid cyme. Thus, the scorpioid cyme has two rows of flowers, one on either side of the sympodial inflorescence axis. (For another example, see the photograph of a forget-me-not scorpioid cyme on the Flowers page.)

Root branching
Like shoots, roots can branch apically or laterally. While apical branching in roots is similar to apical branching in stems, lateral branching is distinct. Notably, roots have originated at least twice in vascular plants, separately in the lycophytes and euphyllophytes.
Root apical branching (dichotomous branching)
Apical or dichotomous branching in roots is similar to apical branching in stems. Dichotomizing roots are found in the lycophytes and are sometimes considered a synapomorphy for that group; however, recent research indicates that apical root branching occurred in some extinct euphyllophytes, even if it is not found in modern euphyllophytes (living ferns, horsetails, and seed plants). Lycophyte roots are sometimes also called dichopodial (Greek, dicho- + Latin, podium = in two foot).
Note: Many plants with apical stem branching lack true roots, including the extinct protracheophytes and early vascular plants (like Agalophyton, illustrated above on this page), as well as the living whisk ferns (Psilotum). These plants are anchored with rhizoids, which are simple thread-like structures. Roots, in contrast, are organs made up of concentric layers of tissues.

Root lateral branching
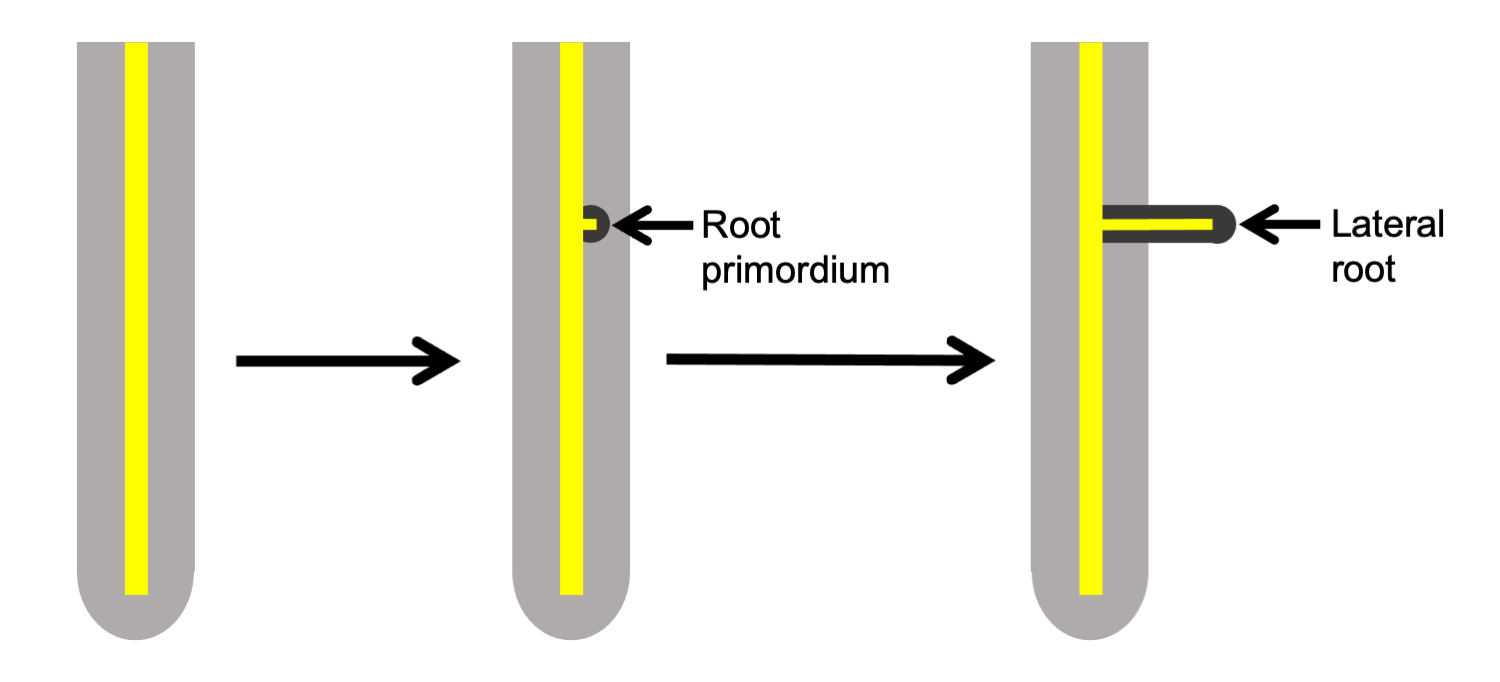
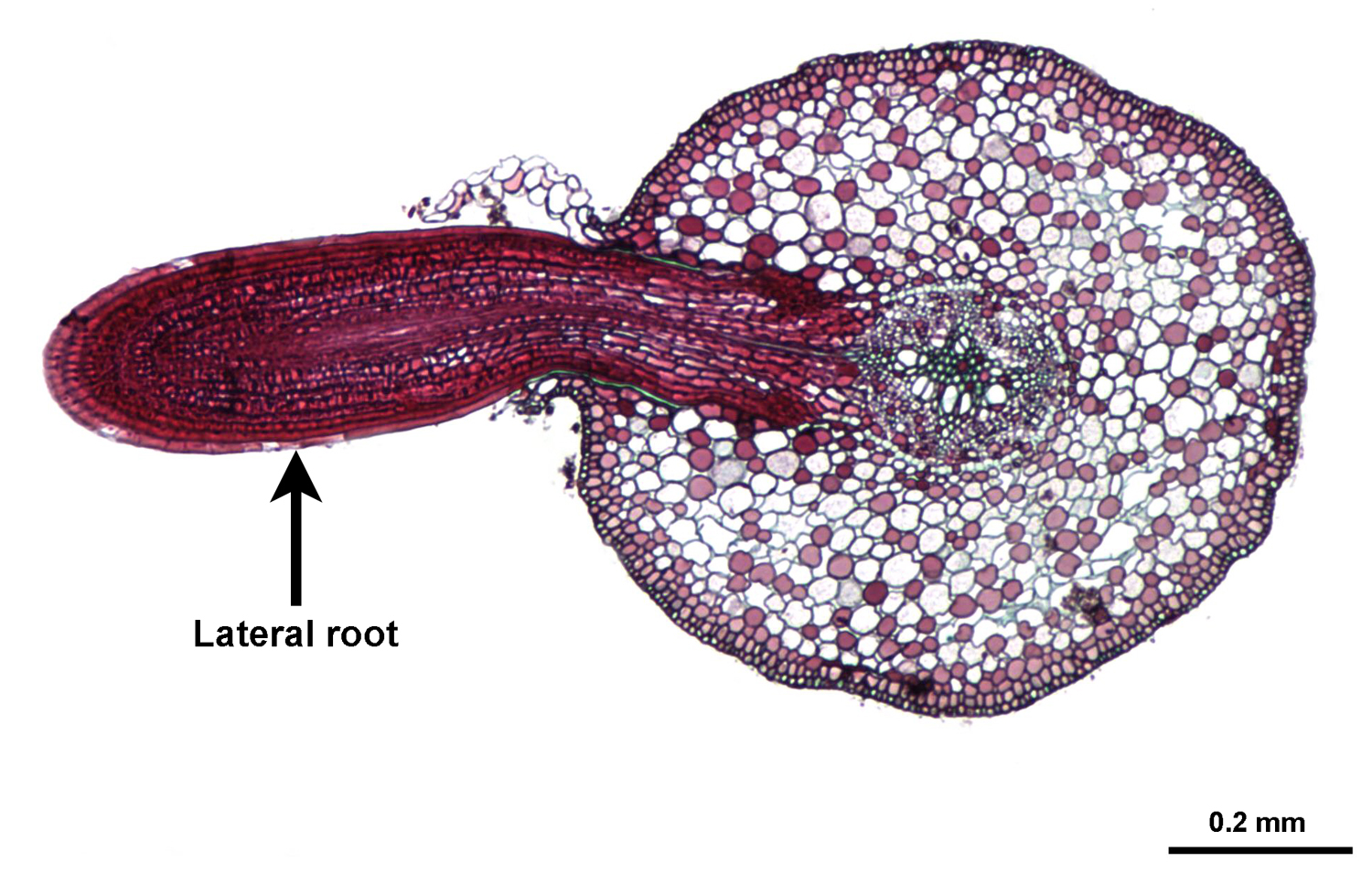
In many euphyllophytes, roots are monopodial and branch laterally. Whereas shoot lateral branches arise exogenously (in other words, from lateral buds on the surface of the stem), root lateral branches arise endogenously (in other words, from within the root itself). Lateral roots begin development in a region known as the pericycle, which forms the outer sheath of the vascular cylinder (strand of vascular tissue). A lateral root must grow through the cortex and epidermis of the root from which it branches.
Selected references & further reading
Note: Free access is provided by the publisher for items marked with a green asterisk.
Academic articles & book chapters
* Banks, H.P., S. Leclercq, and F.M. Heuber. 1975. Anatomy and morphology of Psilophyton dawsonii sp. n. from the late lower Devonian of Quebec (Gaspé), and Ontario, Canada. Palaeontographica Americana 8: 77–127. Available on Biodiversity Heritage Library: https://www.biodiversitylibrary.org/page/10692435
Buys, M.H., and H.H. Hilger. 2003. Boraginaceae cymes are exclusively scorpioid and not helicoid. Taxon 52: 719–724. https://doi.org/10.2307/3647346
* Chomicki, G., M. Coiro, and S.S. Renner. 2017. Evolution and ecology of plant architecture: integrating insights from the fossil record, extant morphology, developmental genetics and phylogenies. Annals of Botany 120: 855–891. https://doi.org/10.1093/aob/mcx113
* Donoghue, M. 1981. Growth patterns of woody plants with examples from the genus Viburnum. Arnoldia 41: 2-23. PDF available from the Arnold Arboretum (Harvard): http://www.arnoldia.arboretum.harvard.edu/pdf/articles/1111.pdf
* Gola, E.M. 2014. Dichotomous branching: the plant form and integrity upon the apical meristem bifurcation. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2014.00263
Hetherington, A.J., and L. Dolan. 2018. Stepwise and independent origins of roots among land plants. Nature 561: 235–238. https://doi.org/10.1038/s41586-018-0445-z Read free on PubMed (PMCID: PMC6175059): https://pubmed.ncbi.nlm.nih.gov/30135586/
Hetherington, A.J., C.M. Berry, and L. Dolan. 2020. Multiple origins of dichotomous and lateral branching during root evolution. Nature Plants 6: 454–459. https://doi.org/10.1038/s41477-020-0646-y
* Matsunaga, K.K.S., and A.M.F. Tomescu. 2016. Root evolution at the base of the lycophyte clade: insights from an Early Devonian lycophyte. Annals of Botany 117: 585–598. https://doi.org/10.1093/aob/mcw006
Stevenson, D.W. 2020. Observations on vegetative branching in cycads. International Journal of Plant Sciences 181: 564–580. https://doi.org/10.1086/708812
* Stopes, M.C. 1910. Adventitious budding and branching in Cycas. New Phytologist 9: 235–241. https://doi.org/10.1111/j.1469-8137.1910.tb05571.x
* Vasco, A., R.C. Moran, and B.A. Ambrose. 2013. The evolution, morphology, and development of fern leaves. Frontiers in Plant Science 4: 345. https://doi.org/10.3389/fpls.2013.00345
Books & textbooks
Bierhorst, D.W. 1971. Morphology of vascular plants. The Macmillian Company, New York, New York.
Foster, A.S., and E.M. Gifford. 1974. Comparative Morphology of Vascular Plants, 2nd ed. W.H. Freeman and Co., San Francisco.
Simpson, M.G. 2010. Plant Systematics, 2nd ed. Academic Press, Burlington, Massachusetts.
Taylor, T.N., E.L. Taylor, and M. Krings. 2009. Paleobotany, the Biology and Evolution of Fossil Plants, 2nd ed. Academic Press.
Blogs, magazines, websites
* Aiello, A.S., and M.S. Dosmann. 2019. Déjà vu viburnums: A world away but close to home. Arnoldia 76: 14–25. https://arboretum.harvard.edu/stories/deja-vu-viburnums-a-world-away-but-close-to-home/
* Hetherington, S. 2020. Unearthing the complex evolutionary origins of root branching. Nature Research Ecology & Evolution Community. https://natureecoevocommunity.nature.com/posts/65945-unearthing-the-complex-evolutionary-origins-of-root-branching
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen or J.R. Hendricks are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
First released 13 January 2021; last updated 24 August 2021.



