Section contents:
Feature image: A bee on an echinacea capitulum (a group of small flowers making up a flowering head), Minns Garden, Cornell University, Ithaca, New York, U.S.A. Credit: E.J. Hermsen (DEAL).
Introduction
One of the major factors that accounts for the great diversity of floral structures and flowering plant species is pollination. Flowers are not colorful or nicely scented in order to please people, although certainly these attributes have proven to be an evolutionary advantage for some plants in the Anthropocene. Rather, one of the primary purposes of flowers is to attract animal pollinators so that pollination and fertilization can occur. Although we can only estimate, over 85% of the world's flowering plant species may be animal pollinated (see here), and animal pollination is very important to the production of certain crops, like cocoa (Theobroma cacao); apples (Malus domestica); cherries, apricots, peaches, and plums (Prunus spp.); blueberries and cranberries (Vaccinium spp.); and melons, squashes, and pumpkins (Citrullus, Cucumis, and Cucurbita) (read more here). While bees (particularly honeybees, Apis mellifera) are the most important pollinators of crop plants, many other types of animals serve as pollinators, including other insects, mammals, and birds. Flowering plants that are not animal-pollinated are often wind-pollinated, and some have pollen delivered by water. Other flowers are self-pollinated.
Floral features may be aligned with the attributes of target pollinators, such as their weight, physical capabilities, visual capabilities, and sense of smell. Pollinators may receive a reward from a flower, often food in the form of nectar or pollen, for visiting. The concept of pollination syndromes or pollinator syndromes attempts to generalize which floral traits correlate with specific categories of pollinators (e.g., beetles, bees, bats, etc.). While pollination syndromes can be useful guidelines, only detailed observation and study can establish which potential pollinators actually visit a given type of flower, and which type of pollinator effectively delivers pollen so that sexual reproduction can occur. Furthermore, many flowers may be pollinated by more than one type of pollinator.
Pollination Syndromes
| Bats | Bees | Beetles | Birds | Butterflies | Carrion flies | Nocturnal moths | Wind | |
|---|---|---|---|---|---|---|---|---|
| Name for pollination syndrome | Chiropterophily | Melittophily | Cantharophily | Ornithophily | Psychophily | Saprophily | Phalaenophily | Anemophily |
| Color | Variable, often dull | Bright & variable, may have UV patterns | Dull (brown, green, white) | Orange, red, yellow | Bright & variable | Pale or dull (green, maroon) | Pale/dull (white) | Dull |
| Shape | Bowl, bottle-brush, etc. | Bell, landing platform, tube, etc. | Bowl | Funnel/tube, bottle-brush, etc. | Landing platform, tube, etc. | Funnel, trap, etc. | Tube, etc. | - |
| Symmetry | Regular or irregular | Regular or irregular | Regular | Regular or irregular | Regular | Regular or irregular | Regular | Regular |
| Reward | Floral tissue, nectar, pollen | Nectar, oil, pollen, resin | Floral tissue, nectar, pollen | Nectar | Nectar | None | Nectar | None |
| Nectar guides | No | Sometimes | No | No | Sometimes | No | No | No |
| Odor | Medium, like fruit or spoiling fruit | Weak, pleasing | None or strong, fruit-like or unpleasant | None | Weak | Strong & disgusting | Medium to strong, sweet | None |
| Other notes | Open & produces scent at night; large flowers | Bees see UV, but do not see red | May produce heat | - | - | - | Produces scent at night | Anthers, stigmas exposed; petals may be absent/small |
Pollination syndromes chart (above). Floral attributes associated with different types of pollinators. Under symmetry, "regular" means that a flower has radial symmetry; "irregular" means that a flower has bilateral symmetry. Note: If you are using a small device and cannot see the whole table, you can scroll horizontally to see additional columns. Modified from Pollinator Syndrome Traits Table at the USDA Forest Service Celebrating Wildflowers website and Rosas-Guerrero et al. (2014), table S1.
Pollination in the fossil record
Pollination is difficult to study directly in the fossil record. After all, flowers are ephemeral, and the act of pollination is fleeting. We can use structural and phylogenetic evidence from modern plants to help us infer the relationships between ancient plants and their pollinators. For example, flowers with nectaries, or nectar-secreting structures, are indicators of animal pollination, since nectar serves as a reward for some types of pollinators. Features of the pollen itself, such as pollen grain size and tendency to form clumps, may indicate whether pollination was likely wind- or animal-mediated. Animal fossils sometimes provide indirect evidence for pollination, such as adaptations for nectar-feeding. Pollen preserved in the digestive tract of fossil animals and coprolites (fossil poop) containing or composed of pollen may also be an indicator of animal pollination, since pollen is the reward some animals receive for visiting a flower.
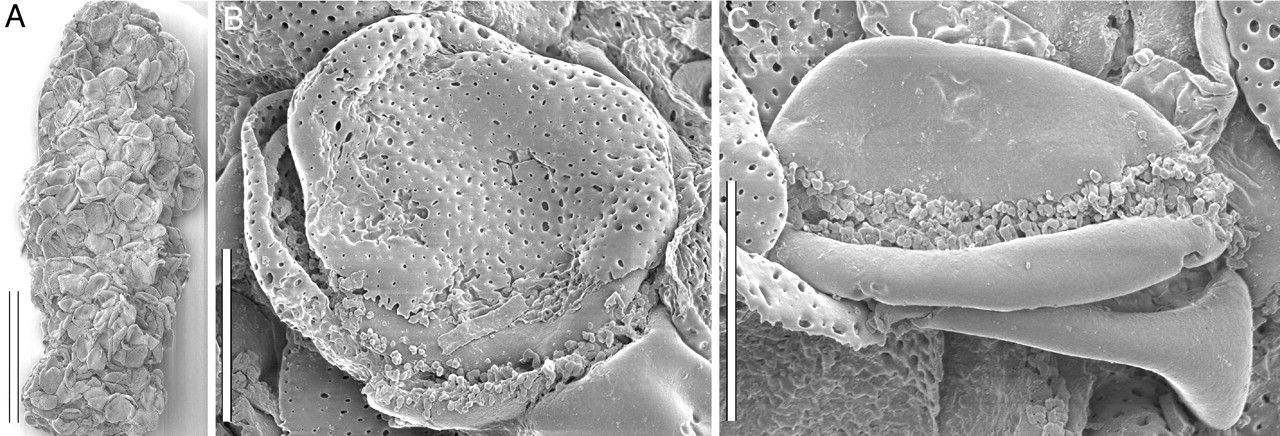
Pollen mass possibly representing a coprolite. Scanning electron photomicrographs of a possible coprolite made up of pollen from the Early Cretaceous of Portugal. Left (A): Entire mass/possible coprolite, scale bar = 150 microns. Center & Right (B-C): Details of individual pollen grains from the coprolite, scale bars = 15 microns. Copy of Figure 3 from Friis et al. (2004), Araceae from the Early Cretaceous of Portugal: Evidence of the emergence monocotyledons. PNAS 101: 16565-16570. Copyright 2004 National Academy of Sciences. Reproduced here following PNAS rights & permissions.
Unbelievable as it may seem, there is some relatively direct evidence for pollination in the fossil record. While the orchid fossil record can perhaps best be described terrible (especially when considered relative to modern orchid diversity), some insects preserved in amber have pollinia (masses of pollen, sometimes with other associated structures) attached to them (for examples, see here, here, and here). Although these insects were not preserved in the act of depositing pollen on a stigma, the attached pollen clumps strongly indicate that the insects were involved in visiting and transferring pollen amongst flowers.
Insect pollination (entomophily)
Insects are also the most important group of animal pollinators, and a recent estimate suggests that there are over 5 million species of insects alive today. Common insect pollinators include bees, wasps, flies, beetles, butterflies, and moths. Insect pollination in seed plants likely appeared in the Paleozoic. Entomophily has been documented in cycads and some gnetophytes among living gymnosperms. It also likely occurred in some extinct seed plant groups, like cycadeoids. Some of the earliest angiosperms were probably insect pollinated, and insect pollination of angiosperms was certainly present in the Cretaceous (two Late Cretaceous examples are provided later on this page).
The first known appearance of major insect pollinator groups in the fossil record varies. Fossil evidence for the orders that include flies (Diptera), beetles (Coleoptera), moths and butterflies (Lepidoptera), and ants, bees, and wasps (Hymenoptera) predates the first appearance of angiosperms in the fossil record. Early Cretaceous tangle-veined flies (Nemestrinidae) are the oldest insect fossils showing structural modifications to their mouthparts and wings that appear to be specifically for procuring food from flowers (see here). Bees, a clade within order Hymenoptera and one of the most important groups of insect pollinators, are relatively young. The oldest fossil with bee-like characteristics, Melittosphex burmensis, is preserved in Burmese amber from the Early Cretaceous and is about 100 million years old. The fossil exhibits a mixture of features of bees and related wasps. It has adaptations (e.g., branching hairs) that suggest that it gathered pollen (read more here and here).

Sweat bees (family Halictidae) preserved in amber. Typically, sweat bees feed on nectar and pollen. Left: Nesagapostemon moronei (Miocene, Dominican Republic). Right: Oligochlora semirugosa (Miocene, Dominican Republic). Credits: Nesagapostemon moronei, MACT-1172, holotype, and Oligochlora semirugosa, KU-DR-21, holotype (Michael S. Engel, via Wikimedia Commons, CC BY 3.0). Specimens published by Engel (2009) ZooKeys 29: 1-12. Images modified from originals.
Insect vision & flower color
Floral colors and patterns may be correlated with the time of day when the target insects are active, and often take advantage of insects' visual capabilities. For example, flowers pollinated by nocturnal insects may be open at night and have duller colors, whereas flowers targeting insects active during the day may be more brightly colored. Flowers may also have patterns, called honey guides or nectar guides, that point insects toward the center of the flower, where pollen and other rewards await.
Many insects have color vision that covers a different range of the light spectrum than human vision does. Often, they can see colors in the ultraviolet (UV) portion of the spectrum. Some flowers have patterns that are visible only in the UV range. Thus, these patterns are visible to insects, but invisible to humans! These patterns can be revealed if flowers are illuminated with light in the UV spectrum. Under UV light, portions of a flower that absorb light in the UV spectrum will appear to be dark or black, whereas portions of a flower that reflect UV will appear lighter.
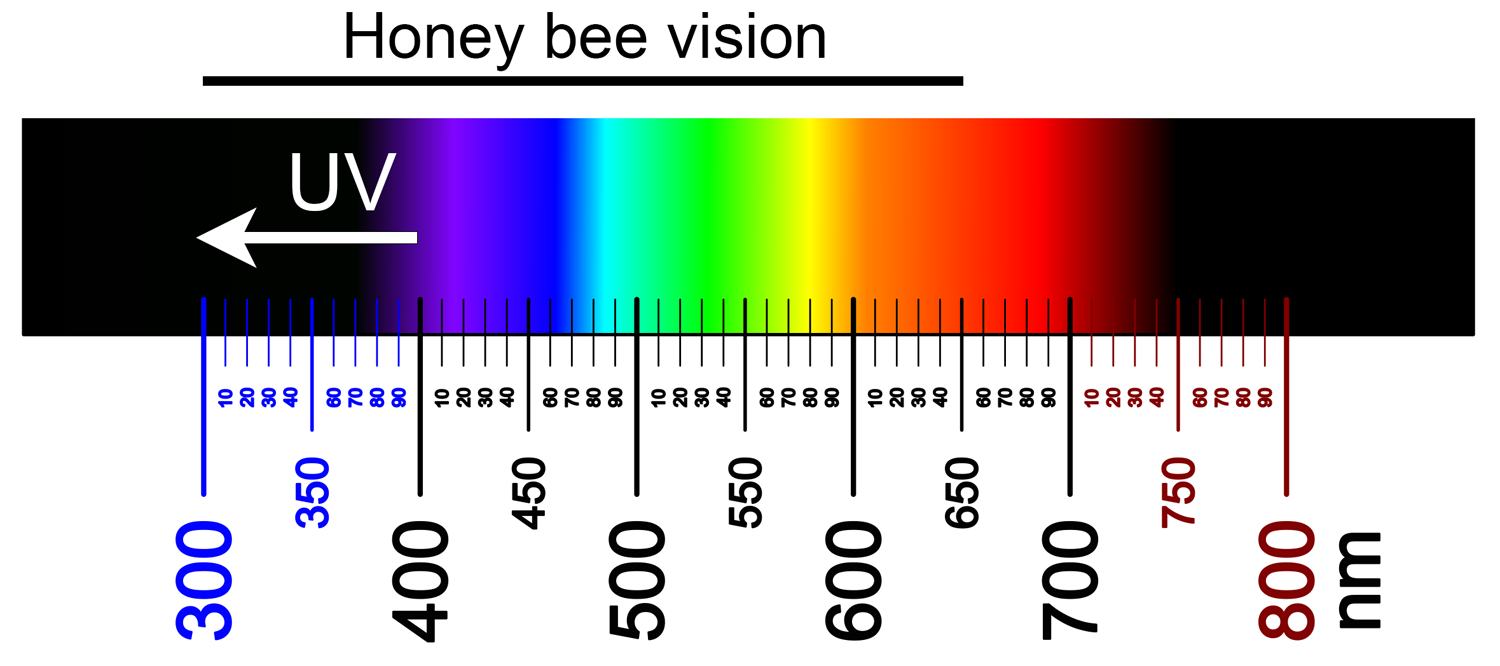
Visual spectrum of honey bees vs. humans. Honey bees (Apis mellifera) can see light in about the 300–650 nm range of wavelengths, with peaks in the UV, blue, and green wavelengths. Sources vary, but the range visible to humans is usually considered to be roughly 400–700 nm (from 380–400 nm on the violet end of the spectrum up to 700–750 nm on the red end of the spectrum), with peaks in the blue, green, and red wavelengths (see here and here). UV light is light in the 10–400 nm range. Image credit: Color spectrum (Fulvio314, via Wikimedia Commons, CC BY-SA 4.0). Image modified from original.
Video showing a capitulum under normal and UV light. A capitulum is a group of tiny flowers that together looks like a single flower; it is typical of the aster family (Asteraceae). On the left is a capitulum shown under normal daylight; note that it is uniformly yellow. On the right, the same capitulum is shown under UV. The black areas are absorbing UV light, whereas the light yellow areas are reflecting UV light. Note: This video has no narration. Credit: See the invisible (Orion17, via YouTube).
While examining flowers under UV light can reveal hidden patterns, it does not show us how the world really looks to an insect that can see light in the UV portion of the spectrum. The black areas that absorb UV light would not necessarily appear dark to an insect. This is because the color of an object is determined by the portion of the visible light spectrum that it reflects, not the portion that it absorbs. For example, an object that appears yellow reflects color in the yellow part of the spectrum (approximately 570–590 nm) and absorbs other wavelengths of visible light. Areas that appear light-colored under UV light reflect UV. This means that to an insect, the color in these areas includes a portion of the UV spectrum.
To complicate things further, some insects cannot see the entire spectrum of light visible to humans. Bees cannot see much of the red spectrum, so this range of colors is subtracted out in "bee vision." On the other hand, some butterflies can see the entire human visible light spectrum plus UV, and can detect subtle differences in color that humans cannot see (read more here). Thus, flower colors and patterns may appear vastly different to insects than they appear to humans.
Some have attempted to approximate how flower colors would actually appear to insects using photography under different types of light and digital manipulation (read more here).
Video showing the colors of flowering plants as seen by humans, bees, and butterflies. Note: This video has background music, but no narration. Credit: Insecta Spectra (Video by Robin Noorda, images by Dr. Klaus Schmidtt, Tropism, via YouTube).
Rewards
Rewards for pollinators are typically food rewards, in the form of floral tissue, pollen, and/or nectar. Nectar is produced by specialized tissue or floral structures known as nectaries. Beetles may sometimes feed on floral tissue or pollen, whereas butterflies and moths are rewarded with nectar. Bees may be after pollen and nectar. Honeybees (Apis mellifera), for example, have specialized pollen baskets on their rear legs where they can pack pollen to take back to the hive.
Rewards may sometimes be more unusual or specialized. For example, some flowers in the mangosteen family (Clusiaceae) reward their pollinators, stingless bees (Family Apidae, Tribe Meliponini), with resin that they can use to construct their nests. Similar flowers, called Paleoclusia chevalieri, have been discovered in Upper Cretaceous (ca. 90 Ma) sediments from New Jersey, U.S.A. A stingless bee in amber (Cretotrigona prisca) was found in younger Upper Cretaceous (66–70 Ma?) deposits from New Jersey. If the Paleoclusia flowers produced resin as a reward, these finds suggest that this specialized plant-pollinator relationship between some members of the mangosteen family and stingless bees could be very ancient, originating sometime between 90 and 66 million years ago!
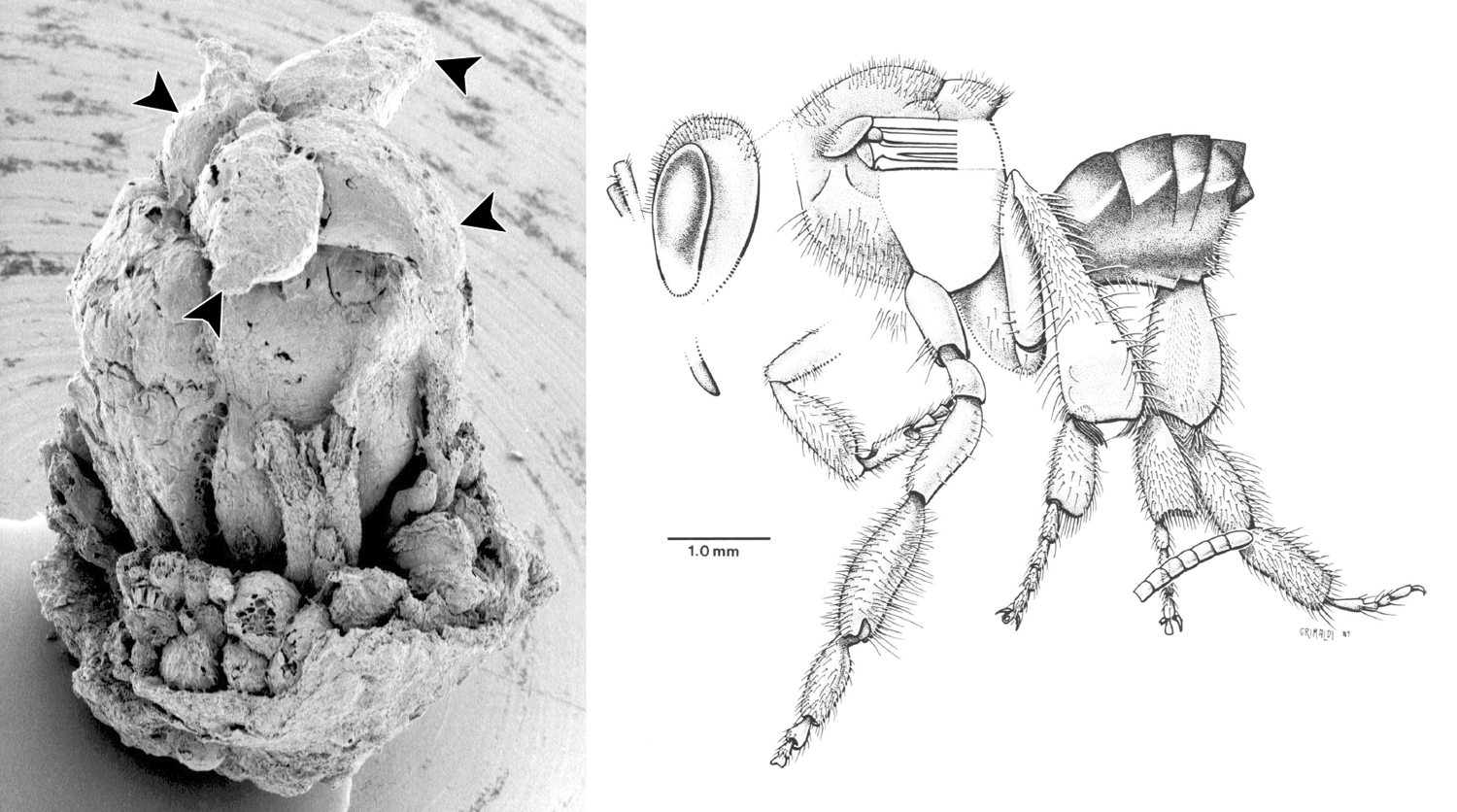
Cretaceous Clusiaceae flowers and stingless bees. Left: Scanning electron photomicrograph of a flower of Paleoclusia chevaieri (Late Cretaceous, New Jersey, U.S.A.). The stigma lobes (indicated by arrowheads) sit on top of the ovary. Remnants of the androecium and perianth can be seen at the base of the ovary. Right: Drawing of Cretotrigona prisca, a stingless bee preserved in amber (Late Cretaceous, New Jersey, U.S.A.). Credits: Paleoclusia chevalieri, CUPC 1203, CUPC image 6052 (copyright 2006 CUPC, via plantsystematics.org, used with permission, terms of use); Drawing of Cretotrigona prisca (from Michener & Grimaldi 1988, The oldest fossil bee: Apoid history, evolutionary stasis, and antiquity of social behavior. PNAS 85: 6424-6426. Reproduced here following PNAS terms rights & permissions). Images modified from originals.
Deception
Deceptive flowers lure pollinators with the promise of something desirable, but instead take advantage of them while often providing them with nothing in return. One typical type of deception involves mimicry of disgusting things to attract flies, like carrion (dead meat), fungus, or dung. These so-called carrion flowers often have dull colors; they may be brownish, maroon, greenish, or mottled. Their scent can be nauseating. Sometimes they are also thermogenic or trap their pollinators (see below).
Examples of carrion flowers are toadshade (Trillium sessile) and carrion plant (Stapelia gigantea). In the arum family (Araceae), an entire inflorescense (group of flowers) can form a single carrion-mimicking structure. The flowers are tiny and grouped on a column called a spadix; the flowers and spadix are surrounded by a petal-like hood, the spathe. Examples of carrion flowers in the arum family include the titan arum (Amorphophallus titanum) and other members of the genus Amorphophallus, as well as the evocatively named dead horse arum lily (Helicodiceros muscivorus). Jack-in-the-pulpit (Arisaema triphyllum), a member of the arum family native to eastern North America, mimics fungus and is pollinated by fungus gnats.
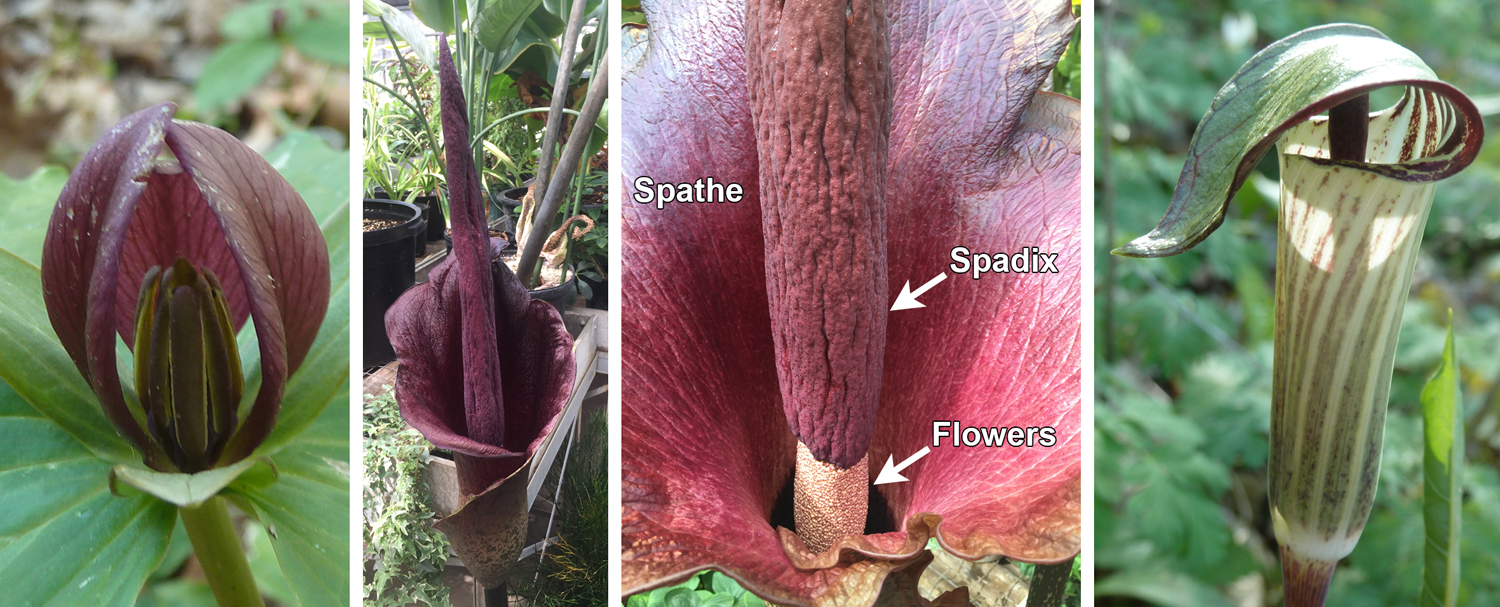
Carrion and fungus-mimicing floral structures. Left: Toadshade (Trillium sessile), a carrion flower. Center panels: Konjac (Amorphophallus konjac), showing the flowering structure from the outside and the interior. Note that the flowers are small and clustered at the base of a pillar known as the spadix. Flowers and spadix are surrounded by a large, maroon spathe. Right: Jack-in-the-pulpit (Arisaema triphyllum), showing the spathe (the hood-like structure) that encloses a group of flowers; jack-in-the-pulpit is pollinated by fungus gnats. All images by E.J. Hermsen (DEAL).
Mimicry comes in other forms. Sexual deception is known in certain orchids. These flowers mimic female bees or wasps, tricking males to attempt to mate with them. In the process, the male insect picks up clumps of pollen that it can transfer to another flower.
Video showing a male wasp attempting to mate with a sexually deceptive orchid. Credit: Orchid's sexual deception triggers ejaculation (New Scientist via YouTube).
Structural features
In addition to color and reward, structural features may encourage or discourage certain types of insect pollinators. Orchids are famous for their structural modifications, which sometimes target very specific pollinators. Darwin's orchid (Angraecum sesquipedale), which grows on the island of Madagascar, is one such orchid. This orchid has a very long spur, or tube, with nectar at the bottom; the tube can be up to about 11 inches (28 cm) long. In his 1862 book on orchid pollination, Charles Darwin predicted that a moth with a correspondingly long proboscis (tube-like mouthpart) must pollinate the orchid (see here). The pollinator, Morgan's spinx moth (Xanthopan morganii) was not discovered until the early 20th century, and was only conclusively proven to be the pollinator in the 1990's (see here).
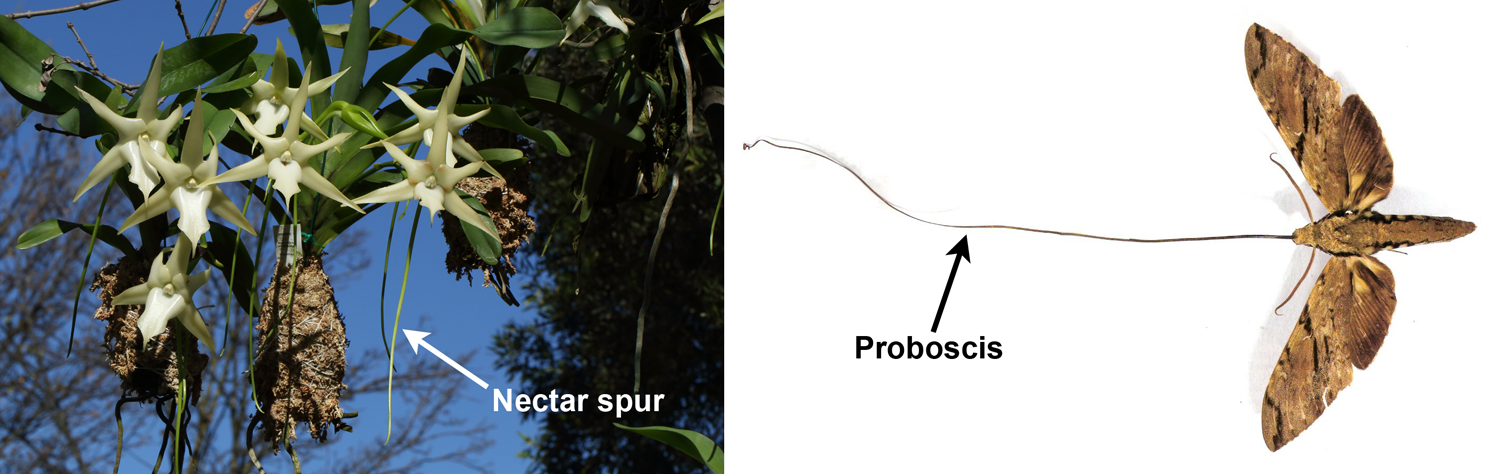
Specialized flower-pollinator structural features. Left: Darwin's orchid (Angraecum sesquipedale) showing long nectar spur, which has a relatively small amount of nectar at the bottom. Right: Morgan's spinx moth (Xanthopan morganii) showing long proboscis (mouthpart) that can reach the nectar in the spur. Credits: Angraecum sesquipedale (Wilferd Duckitt, via Wikimedia Commons, CC BY 2.0); Xanthopan morganii (Esculapio, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals for DEAL.
Anthers, the pollen-producing structures in flowers, often open by long slits to release their pollen. However, some flowers have anthers that open by tiny apical pores or slits. These flowers are pollinated by sonicating bees, like bumblebees (Bombus). Bumblebees can vibrate their bodies at the right frequency to free the pollen from the anthers in a process known as buzz pollination. Some well known crop plants have flowers adapted for buzz pollination, including members of the nightshade family (Solanaceae)—like potato and tomato (Solanum spp.)—and members of the heath family (Ericaceae), like blueberries and cranberries (Vaccinium spp.).
Video explaining buzz pollination. Credit: This Vibrating Bumblebee Unlocks a Flower's Hidden Treasure (Deep Look, PBS Digital Studios via YouTube).
Thermogenesis (heat production)
Thermogenesis has been documented in members of 11 flowering plant families (see here). Thermogenesis means that a flower or inflorescence generates heat. Thermogenesis is often linked to big, beetle-pollinated flowers, like the giant water lily (Victoria) of South America. Heat is also produced by some plants pollinated by insects attracted to carrion, like the dead horse arum lily (Helicodiceros muscivorus). The relationship between pollination and thermogenesis probably predates the origin of angiosperms, because thermogenesis is known to occur in living cycads, a group that first appears in the Paleozoic. Cycad thermogenesis is associated with pollination by thrips and beetles.
Thermogenesis may play a variety of roles in plant pollination and reproduction that may be of benefit to either plant, pollinator, or both. In some cases, thermogenesis (warmth) may protect the plant and serve as a reward for pollinators. For example, the thermogenic North American eastern skunk cabbage (Symplocarpus foetidus) often blooms when there is still snow on the ground (see video below). Thermogenesis is sometimes associated with deceit (lack of a pollinator reward) and entrapment (trapping of pollinators).
Video showing thermogenesis in skunk cabbage. Credit: Undead zombie flowers of Skunk Cabbage (Plants are Cool, Too! by Dr. Chris Martine, Bucknell University, and co-sponsored by the Botanical Society of America, via YouTube).
Entrapment
Some flowering plants have structural modifications or exhibit movement of structures to entrap pollinators. Entrapment can serve as a mechanism to help enforce cross-pollination. Flowers may lure their pollinators when their stigmas are receptive to pollination, then trap the pollinators until they release their own pollen. Once released, the pollinators can fly to another receptive flower to repeat the pollination and entrapment cycle.
Giant water lilies (Victoria) native to South America are an example of plants that entrap their pollinators. These water lilies utilize thermogenesis and scent to attract beetles that crawl into a chamber in the flower. The flower closes and temporarily traps the beetles, which feed on floral tissues. The beetles are freed the next day after the anthers open to release their pollen, ideally to carry that pollen to another receptive flower. The flower is white when it is receptive to pollination (female phase) and turns pink after trapping its pollinators (male phase). (Read more here.)
Video of a giant water lily (Victoria) blooming. This video is a time-lapse showing the blooming of a giant water lily flower over the course of two days. The flower opens in its white (female) phase, closes to entrap pollinators, turns pink (indicating it is in the male phase), releases its pollen and opens again to free its pollinators, then closes and sinks under the surface of the water. Because this specimen is cultivated in a green house, it is not visited by pollinators. Note: This video has no narration. Credit: Victoria time lapse (Cornell SIPS, via YouTube).
Late Cretaceous flowers from New Jersey, U.S.A., named Microvictoria svitkoana (Svitko's little Victoria), have a floral structure that is similar to that of the living giant water lily, suggesting that they may have employed a similar entrapment mechanism in their pollination system (read more here). Although we cannot observe this directly, perhaps Microvictoria flowers produced heat as well!
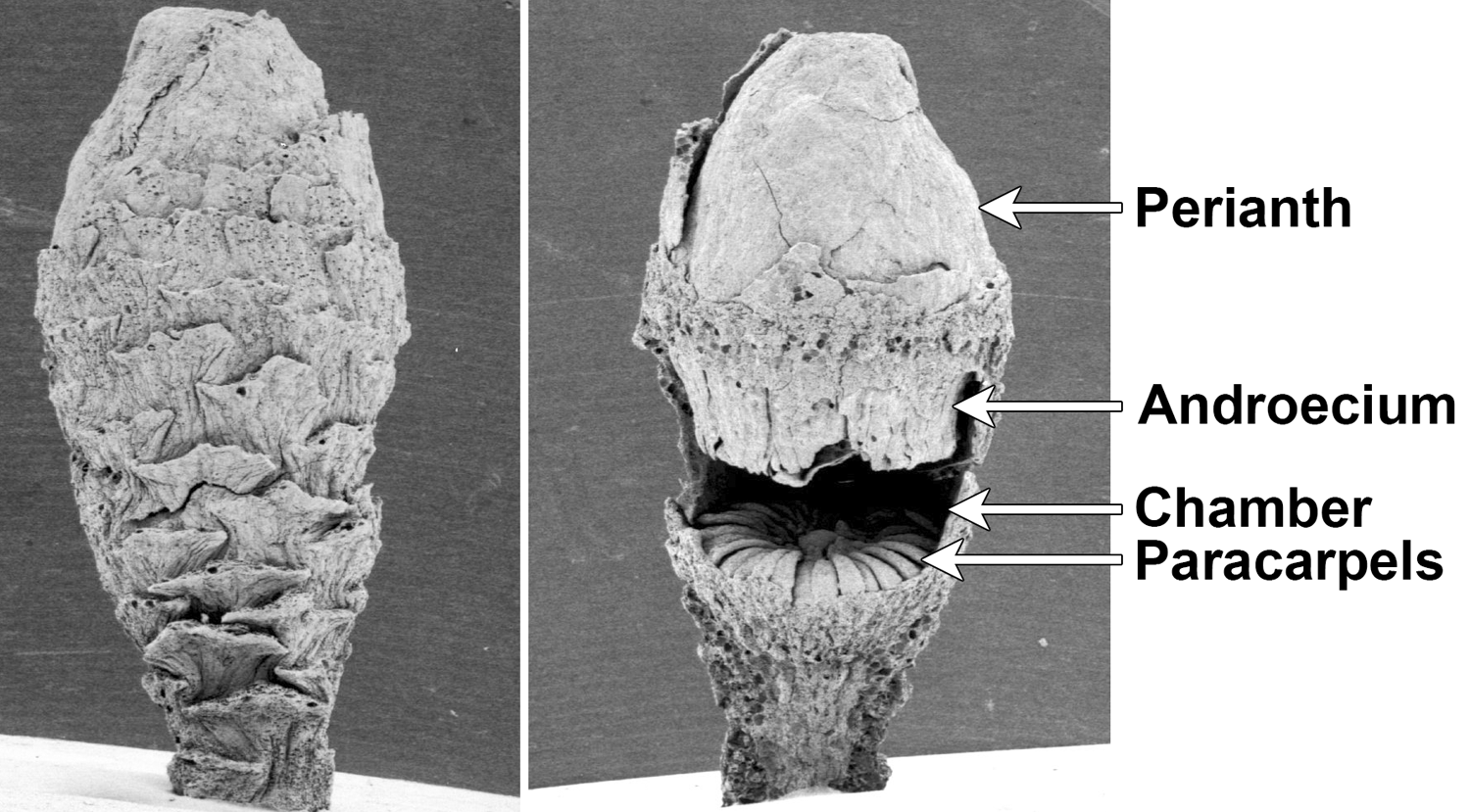
Microvictoria svitkoana, an ancient water lily. Scanning election photomicrographs of a Microvictoria svitkoana flower bud (Late Cretaceous, New Jersey, U.S.A.). Left: Complete flower bud, perianth still closed. Right: Partially dissected flower bud viewed from the side (part of the side of the flower bud has been removed). When the perianth opened, pollinators would have crawled into a small opening through the androecium (fertile and sterile stamens). The paracarpels (sterile appendages) at the base of the chamber would have served as the reward (food) for the pollinators. Credits: Microvictoria svitkoana, specimen CUPC 1475, CUPC image 4543 and CUPC image 4544 (copyright 2005 CUPC, via plantsystematics.org, used with permission, terms of use). Specimen published by Gandolfo et al. (2004) PNAS 101: 8056-8060. Images modified from originals.
Some deceptive carrion and fungus-mimicking plants, like jack-in-the-pulpit (Arisaema triphyllum) and the dead horse arum lily (Helicodiceros muscivorus), mentioned above, also trap their pollinators. Entrapment is not always benign. For example, fragrant water lily (Nymphaea odorata) produces a pool of liquid that covers the stigma of its flower. In order for pollination to occur, pollinators must fall into the pool, where the pollen is washed off of them. Some of the fragrant water lily's pollinators get trapped in the pool and die (read more here, here, and here). Similarly, the fungus gnats that pollinate the jack-in-the-pulpit can be fatally trapped after delivering their pollen, because there is no escape hole at the bottom of the spathe (hood) surrounding flowers of female plants (read more here).
Bat pollination (chiropterophily)
Bat pollination is estimated to have originated over 50 separate times in flowering plants, and is known to occur in over 500 flowering plant species (see here). Bats are relatively large pollinators that are active at night (nocturnal) and, like many mammals, colorblind. As might be expected based on these characteristics, bat-pollinated flowers are often dull-colored, large, and open at night when bats are active. They tend to produce a fruit-like fragrance and nectar as a reward. Examples of bat-pollinated plants include bananas (Musa acuminata), baobabs (Adansonia digitata), durian (Durio), and saguaro cacti (Carnegiea gigantea).
When did bat pollination first evolve? The best evidence comes from our knowledge of bat evolution and relationships based on study of modern bats and the bat fossil record. The oldest known bat fossils are from the Eocene, and molecular phylogenetic evidence suggests an origin for bats near the Cretaceous-Paleogene boundary. Pollinating bats occur in two unrelated families: the American leaf-nosed bats (Phyllostomidae) in the New World, and various lineages of "megabats"—or Old World fruit bats (Pteropodidae)—in the Old World (see here). In the New World, pollinating (nectar-eating) bats are thought to have evolved twice in the Miocene, and are represented by at least one Miocene fossil (see here and here). The fossil record of Old World fruit bats is poor, although the family probably arose in the Paleogene.
Video discussing the role of bats as pollinators of agave. Credit: Love tequila, love pollinating bats! (Smithsonian, via YouTube).
Bird pollination (ornithophily)
Bird pollination has evolved in over 60 families of flowering plants. Birds that pollinate flowers are typically rewarded with nectar. Since pollinating birds have a poor sense of smell, bird-pollinated flowers may have no scent. Structures supporting the flower may be robust if the flower is pollinated by birds that must perch to feed, although hummingbirds can hover. Birds can see in a broad range of the visible spectrum, including in the UV range. Despite this, bird-pollinated flowers are often red and do not tend to have UV patterns like some insect-pollinated flowers. (Read more about bird pollination here.)
When did bird pollination evolve? The fossil record of birds provides clues. The oldest known bird fossil is (arguably) Archaeopterix, from the Jurassic of Germany. However, the oldest evidence for bird pollination may be a fossil bird (Pumiliornis tessellatus) from the middle Eocene Lagerstätte of Messel, Germany. A specimen was found with angiosperm pollen in its digestive tract. The bird also showed skeletal modifications for perching and beak flexibility that may indicate that it was a nectar-feeder.
Today, nectar-feeding and bird pollination primarily involves three specialized groups of birds with differing geographic distributions. In the Americas, these are the hummingbirds (Trochilidae); in the Old World, these include the honey-eaters (Meliphagidae) and sunbirds (Nectariniidae) (see here). Stem-group hummingbirds (Eurotrochilus inexpectatus) with clear adaptations for nectar-feeding and hovering are known from the Oligocene of Germany. Fossil honeyeaters are first known from the Neogene of Australia (see here).

Bird pollinators. Left: A ruby-throated hummingbird (Archilochus colubris), native to North and Central America. Right: Eastern spinebill (Acanthorhynchus tenuirostris), a honeyeater native to Australia. Image credits: Rudy-throated hummingbird (Russ, via Wikimedia Commons, CC BY 2.0); eastern spinebill (JJ Harrison, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Wind pollination (anemophily)
Wind pollination is thought to be very ancient, as it was likely the means of pollination for the earliest seed plants. Some living gymnosperms, like pine (Pinus) and most species of ephedra (Ephedra), are wind-pollinated. Wind-pollinated plants are also present amongst angiosperms, and wind pollination likely evolved early in angiosperm evolution.
Wind-pollinated flowers may be inconspicuous. Petals may be absent, as they are not needed to attract pollinators and may serve as barriers to wind pollination. Flowers are often unisexual. Stigmas and anthers tend to be exposed, and stamens and styles may be long. Large amounts of pollen are often produced. Because of this, wind-pollinated plants (particularly trees) tend to be well represented in fossil pollen spectra, or diagrams showing the relative abundance of pollen types counted in rock or sediment samples. Grasses and certain types of trees, like oaks (Quercus) and walnuts (Juglans), are wind-pollinated. Wind-pollinated plants are often the culprits behind seasonal allergies, or hayfever; for example, ragweed (Ambrosia) is wind-pollinated.

Wind-pollinated flowering plants. Left: Common ragweed (Ambrosia artemisiifolia), showing male and female groups of flowers. Center: A group of staminate (male) flowers of English walnut (Juglans regia). Right: Pistillate (female) flowers of English walnut (J. regia), showing the large, exposed stigmas. Image credits: Ambrosia artemisiifolia (Stefan.Iefnaer, via Wikimedia Commons, CC BY-SA 4.0); Juglans regia male flowers (H. Zell, via Wikimedia Commons, CC BY-SA 3.0); Juglans regia female flowers (Ernest Niedermann-VIEX, via Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Self-pollination (autogamy)
Self-pollination occurs when the pollen is transferred from an anther to a stigma on the same plant. Typically, self-pollination is also used to mean that self-fertilization (fusion of sperm and egg from the same plant) can occur. Not all flowers are capable of self-fertilization because some plants have mechanisms to enforce outcrossing, or cross-pollination and cross-fertilization. These can be physiological (e.g., chemical signaling), temporal (e.g., the stigma is receptive at a different time then when pollen is shed from a flower), or structural (e.g., the relative positions of anthers and stigmas in flowers make self-pollination difficult).
Self-pollination has pros and cons as a strategy. The main benefit is that it is reliable mechanism for achieving pollination. However, one of the major drawbacks is that it leads to homogeneity (lack of variation) in populations. Both of these features, reliability and homogeneity, can be desirable in crop plants. For example, garden peas (Pisum sativum) are self-pollinating.
One commonly self-pollinated wild flower is wild ginger (Asarum), a northern hemisphere member of the birthwort family (Aristolochiaceae). The anthers in the flowers open over the stigmas, releasing their pollen, and cross-fertilization rarely occurs. The bee orchid (Ophrys apifera) is a sexually deceptive orchid found in Europe, North Africa, and the Middle East that is also typically self-pollinated.

Self-pollinating flowers: Left: Garden pea (Pisum sativum). Center: Wild ginger (Asarum canadense). Right: Bee orchid (Ophrys apifera). The bee orchid is also a sexually deceptive flower that is sometimes pollinated by long-horned bees (Eucera). Image credits (left to right): White pea flower (net_efekt, via Wikimedia Commons, CC BY 2.0); wild ginger (E.J. Hermsen, DEAL); bee orchid (Bernard DUPONT, via Wikimedia Commons, CC BY-SA 2.0). Images modified from originals.
Selected references & further reading
Note: Free full text is made available by the publisher for items marked with a green asterisk.
Academic articles & book chapters
Avarguès-Weber, A., T. Mota, and M. Giurfa. 2012. New vistas on honey bee vision. Apidologie 43: 244–268. https://doi.org/10.1007/s13592-012-0124-2
Baker, R.J., O.R.P. Bininda-Emonds, H. Mantilla-Meluk, C.A. Porter and R.A. Van Den Bussche. 2012. Molecular time scale of diversification in New World Leaf-Nosed bats (Phyllostomidae): a phylogenetic perspective. Pp. 385–409 in G.F. Gunnell and N.B. Simmons (eds.), Evolutionary history of bats, fossils, molecules and morphology. Cambridge University Press. https://doi.org/10.1017/CBO9781139045599.012
Boles, W.E. 2005. Fossil honeyeaters (Meliphagidae) from the Late Tertiary of Riversleigh, north-western Queensland. Emu 105: 21–26. https://doi.org/10.1071/MU03024
* Chen, P.-J., F.J. Stewart, and K. Arikawa. 2018. The more, the better? A butterfly with 15 different kinds of light sensors in its eye. Frontiers for Young Minds 6:70. https://doi.org/10.3389/frym.2017.00070
* Crepet, W.L., and K.C. Nixon. 1998. Fossil Clusiaceae from the Late Cretaceous (Turonian) of New Jersey and implications regarding the history of bee pollination. American Journal of Botany 85: 1122–1133. https://doi.org/10.2307/2446345
* Cronk, Q., and I. Ojeda. 2008. Bird-pollinated flowers in an evolutionary and molecular context. Journal of Experimental Botany 59: 715–727. https://doi.org/10.1093/jxb/ern009
Culley, T.M., S.G. Weller, and A.K. Sakai. 2002. The evolution of wind pollination in angiosperms. TRENDS in Ecology & Evolution 17: 361–369. https://doi.org/10.1016/S0169-5347(02)02540-5
Danforth, B.N., and G.O. Poinar, Jr. 2011. Morphology, classification, and antiquity of Melittosphex burmensis (Apoidea: Melittosphecidae) and implications for early bee evolution. Journal of Paleontology 85: 882–891. https://doi.org/10.1666/10-130.1
De Luca, P.A., and M. Vallejo-Marín. 2013. What's the 'buzz' about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16: 429–435. https://doi.org/10.1016/j.pbi.2013.05.002
* Engel, M.S. 2000. A new interpretation of the oldest fossil bee (Hymenoptera: Apidae). American Museum Novitates 3296: 1–11. http://hdl.handle.net/2246/2087
* Engel, M.S. 2009. Two new halictine bees in Miocene amber from the Dominican Republic (Hymenoptera, Halictidae). ZooKeys 29: 1–12. https://doi.org/10.3897/zookeys.29.257
* Fleming, T.H., C. Geiselman, and W.J. Kress. 2009. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104: 1017–1043. https://doi.org/10.1093/aob/mcp197
* Friis, E.M., K.R. Pedersen, and P.R. Crane. 2004. Araceae from the Early Cretaceous of Portugal: Evidence on the emergence of monocotyledons. Proceedings of the National Academy of Sciences, U.S.A. 101: 16565–16570. https://doi.org/10.1073/pnas.0407174101
* Gandolfo, M.A., K.C. Nixon, and W.L. Crepet. 2004. Cretaceous flowers of Nymphaeaceae and implications for complex insect entrapment pollination mechanisms in early angiosperms. Proceedings of the National Academy of Sciences, U.S.A. 101: 8056–8060. https://doi.org/10.1073/pnas.0402473101
Jürgens, A., and A. Shuttleworth. 2016. Carrion and dung mimicry in plants. Pp. 361–386 in M.E. Benbow, J.K. Tomberlin, and A.M. Tarone, eds., Carrion ecology, evolution, and their applications. CRC Press, Boca Raton, FL.
* Klein, A.-M., B.E. Vaissière, J.H. Cane, I. Steffan-Dewenter, S.A. Cunningham, C. Kremen, and T. Tscharntke. 2006. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society B, Biological Sciences 274: 303–313. https://doi.org/10.1098/rspb.2006.3721
* Mayr, G. 2004. Old world fossil record of modern-type hummingbirds. Science 304: 861–864. https://doi.org/10.1126/science.1096856
* Mayr, G., and V. Wilde. 2014. Eocene fossil is earliest evidence of flower-visiting by birds. Biology Letters 10: 20140223. http://dx.doi.org/10.1098/rsbl.2014.0223 Free full text at PubMed Central (PMCID: PMC4046380)
* Micheneau, C., S.D. Johnson, and M.F. Fay. 2009. Orchid pollination: from Darwin to the present day. Botanical Journal of the Linnean Society 161: 1–19. https://doi.org./10.1111/j.1095-8339.2009.00995.x
* Michener, C.D., and D.A. Grimaldi. 1988. The oldest fossil bee: Apoid history, evolutionary stasis, and the antiquity of social behavior. Proceedings of the National Academy of Sciences, U.S.A. 85: 6424–6426. https://doi.org/10.1073/pnas.85.17.6424
Ollerton, J., R. Winfree, and S. Tarrant. 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Poinar, G. 2016. Orchid pollinaria (Orchidaceae) attached to stingless bees (Hymenoptera: Apidae) in Dominican amber. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen 279: 287–293. https://doi.org/10.1127/njgpa/2016/0556
Poinar, G.O., and B.N. Danforth. 2006. A fossil bee from Early Cretaceous Burmese amber. Science 314: 614. https://doi.org/10.1126/science.1134103
* Poinar, G., Jr., and F.N. Rasmussen. 2017. Orchids from the past, with a new species in Baltic amber. Botanical Journal of the Linnean Society 183: 327–333. https://doi.org/10.1093/botlinnean/bow018
Ramírez, S.R., B. Gravendeel, R.B. Singer, C.R. Marshall, and N.E. Pierce. 2007. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature 448: 1042–1045. https://doi.org/10.1038/nature06039
* Rosas-Guerrero, V., R. Aguilar, S. Martén-Rodríguez, L. Ashworth, M. Lopezaraiza-Mikel, J.M. Bastida, and M. Quesada. 2014. A quatitative review of polliantion syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388–400. https://doi.org/10.1111/ele.12224
Stork, N.E. 2018. How many species of insects and other terrestrial arthropods are there on earth? Annual Review of Entomology 63: 31–45. https://doi.org/10.1146/annurev-ento-020117-043348
* Thien, L.B., P. Bernhardt, M.S. Devall, Z.-d. Chen, Y.-b. Luo, J.-H. Fan, L.-C. Yuan, and J.H. Williams. 2009. Pollination biology of basal angiosperms (ANITA grade). American Journal of Botany 96: 166–182. https://doi.org/10.3732/ajb.0800016
Books & textbooks
Evert, R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York. [See Chapter 20.]
Grimaldi, D., and M.S. Engel. 2005. Evolution of the insects. Cambridge University Press, New York.
Websites
* In Defense of Plants: A common plant with an odd pollination mechanism. Posted November 2, 2017. http://www.indefenseofplants.com/blog/2017/10/24/a-common-plant-with-an-odd-pollination-mechanism
* Plant Talk: Inside the New York Botanical Garden. Jack-in-the-pulpit: Pollination by deception. Author: Carol Grace. Posted June 12, 2013. https://www.nybg.org/blogs/plant-talk/2013/06/science/jack-in-the-pulpit-pollination-by-deception/
* USDA Forest Service. Celebrating Wildflowers: Pollinators: Plant Strategies: Entrapment. https://www.fs.fed.us/wildflowers/pollinators/Plant_Strategies/entrapment.shtml
* USDA Forest Service. Celebrating Wildflowers: Pollinators: Pollinator Syndromes. https://www.fs.fed.us/wildflowers/pollinators/What_is_Pollination/syndromes.shtml
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
Last updated 11 June 2020.



