Section contents:
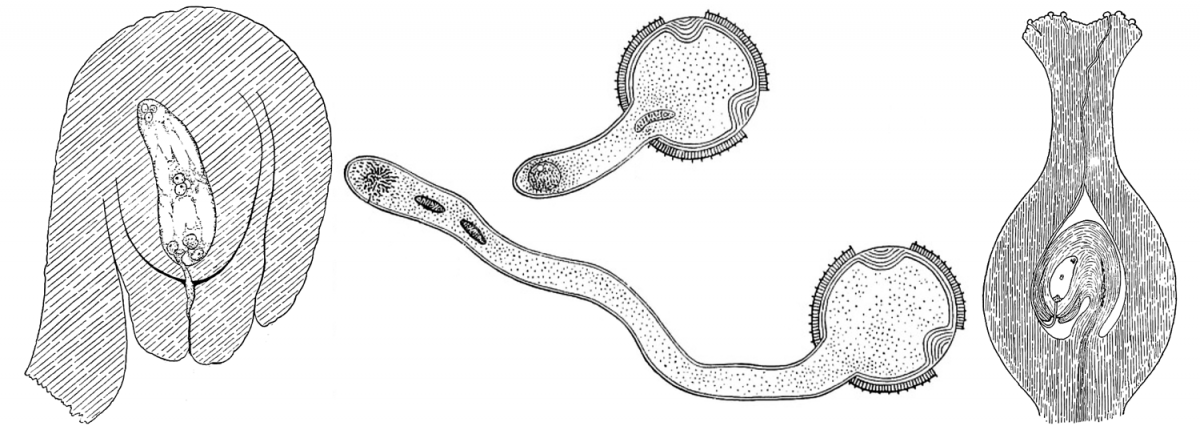
Feature image: Composite image showing fertilization of an angiosperm ovule. Left: Ovule with female gametophyte (megagametophyte or embryo sac) at the time of fertilization. Center: Two stages in growth of the pollen tube from a pollen grain. Right: Longitudinal section of a pistil showing a pollen tube growing from the pollen grain on the stigma, down the style, and into the ovary, where it is fertilizing an ovule. Credits: Figures 231, 118, and 119 from Bergen & Caldwell (1914) Introduction to Botany (no known copyright restrictions). Images modified from originals.
Overview
The angiosperm life cycle, in many ways, follows the basic life cycle pattern for land plants (embryophytes), with modifications characteristic of the seed plant habit (read more here). As in other seed plants, the microgametophyte (male, or sperm-producing gametophyte) is highly simplified and called a pollen grain.The megagametophyte (female, or egg-producing, gametophyte) develops within the ovule (immature seed). The pollen grain must be released and transported to the ovule-bearing structure before fertilization can occur. However, angiosperm pollen grains, megagametophytes, and fertilization differ from those of other seed plants in several important ways.
Angiosperm pollen is produced in the anther, a floral structure that is typically made up of two pairs of fused microsporangia (microspore-producing sporangia, also called pollen sacs). Pollen grains of angiosperms are the most simplified in all of seed plants. At maturity, each pollen grain includes three cells: one tube cell (the cell that forms the pollen tube) and two sperm.
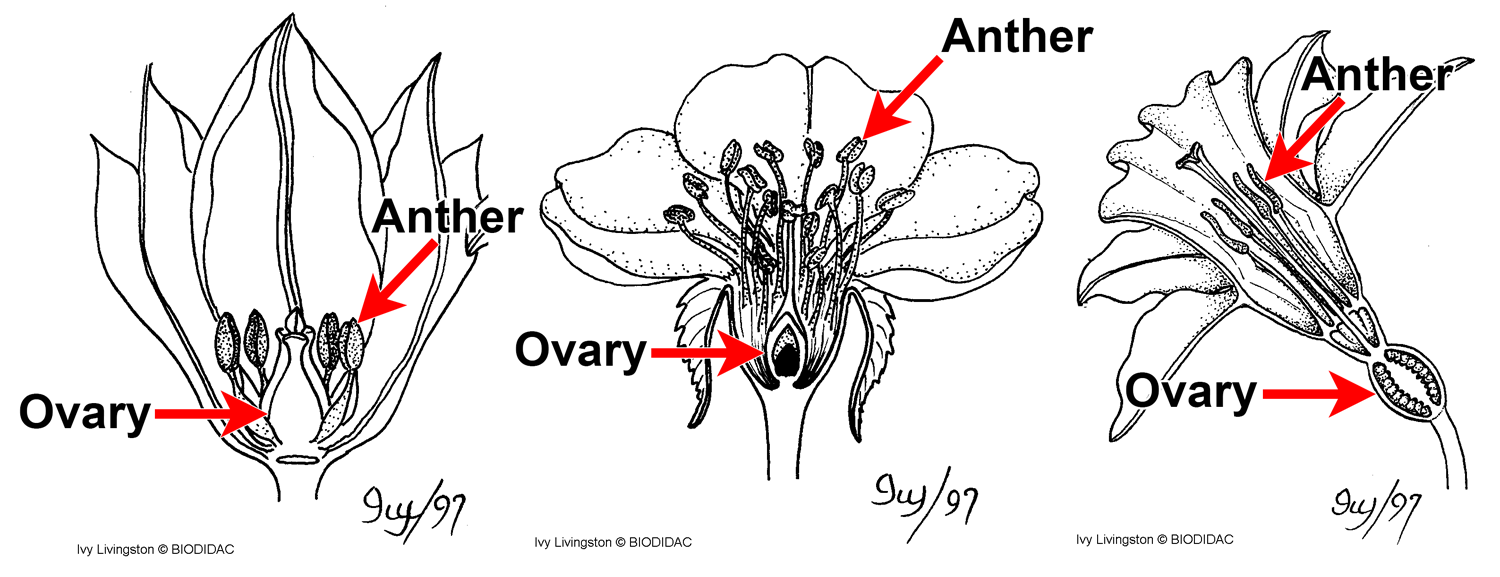
Flowers showing ovaries and anthers. In angiosperms, the ovules that contain the megagametophytes (female or egg-producing gametophytes) are enclosed in an ovary. The pollen grains are produced in microsporangia (pollen sacs) that are part of the anther. Credit: Hypogynous flower, perigynous flower, and epigynous flower (Drawings by Ivy Livingston, BIODIDAC, CC BY-NC 4.0). Drawings modified from originals.
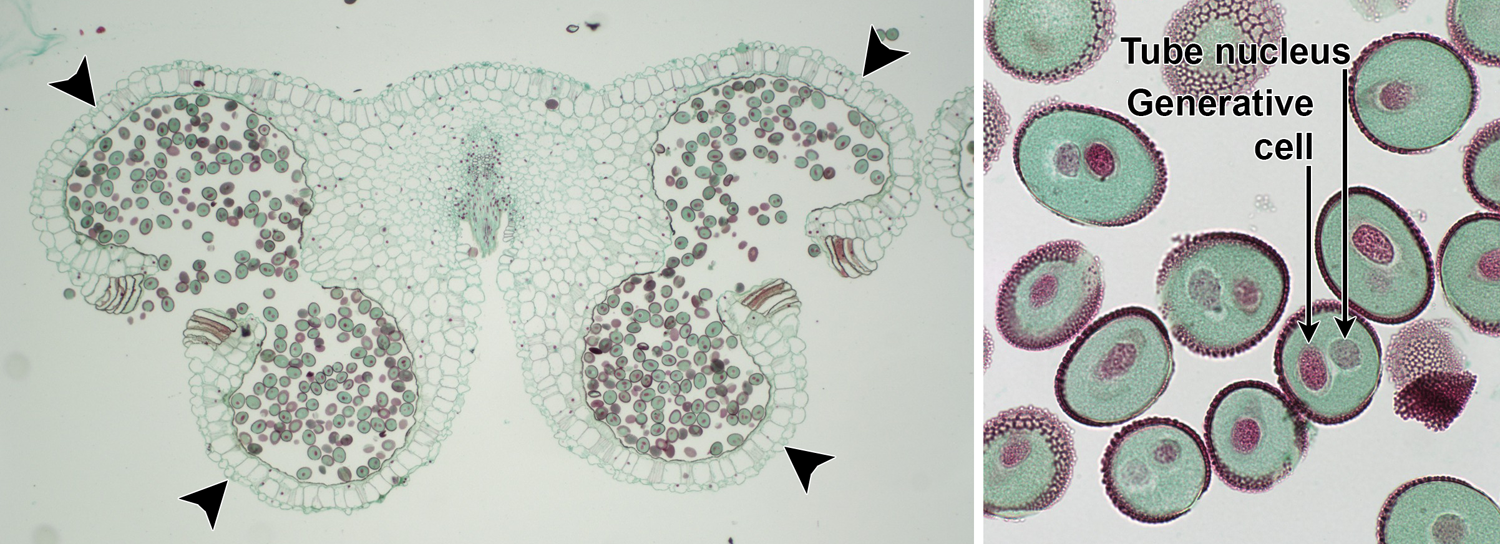
Lily anther and pollen. Left: Transverse section of a Lily (Lilium) anther showing the typical angiosperm arrangement of four pollen sacs (microsporangia) in two pairs (each pollen sac is indicated by an arrowhead); the sacs contain 2-celled pollen grains. The anther has dehisced (opened) and is ready to release the pollen. Right: Detail of two-celled pollen grains. The tube cell will elongate to form the pollen tube, whereas the generative cell will divide to yield two sperm. Credits: Lilium anther and pollen (CUPAC, copyright 2011 Cornell University Plant Anatomy Collection, used with permission). Images modified from originals.
The pollen grains of angiosperms cannot land directly on the ovules because the ovules are enclosed in a floral structure called an ovary (the ovary is indicated in the figure at the top of the page). Thus, the pollen grains land on a specialized surface (the stigma), where they germinate. Angiosperms are siphonogamous (Greek, siphon + gamia = tube union), meaning that their sperm are not motile (i.e., lack flagella and cannot swim) and are delivered to the egg by means of a pollen tube. The pollen tube must grow from the pollen grain on the stigma, down the style, and into the ovary to make contact with an ovule.

Lily flower and germinated pollen. Left: Close-up of lily (Lilium) flower showing open anthers and the stigma, the surface on which pollen will land. Right: Scanning electron micrograph (SEM image) of germinated lily (Lilium) pollen grains showing pollen tubes growing out of apertures (thin areas) in the pollen walls. Credit: Closeup of stamen and stigma of Lilium (Subhrajyoti07, Wikimedia Commons, CC BY-SA 4.0); SEM micrograph of lily pollen tubes (Neutr0nics, Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Unlike gymnosperm ovules, angiosperm ovules often have a double integument, or two distinct integuments that surround the nucellus (megasporangium, the megaspore-producing sporangium) in which the megagametophyte develops. Angiosperm ovules are often folded over on themselves. The ovule is attached to the inner ovary wall by a stalk called a funiculus or funicle.
The megagametophytes (female or egg-producing gametophytes) of angiosperms are also called embryo sacs. The megagametophytes are highly simplified compared to those of other seed plants. Most angiosperm megagametophytes are made up of only seven cells, although they may have as few as four cells. One egg cell is present per megagametophyte, and there is no archegonium. (The archegonium is a multicellular structure that surrounds the egg cell in many other plant groups).
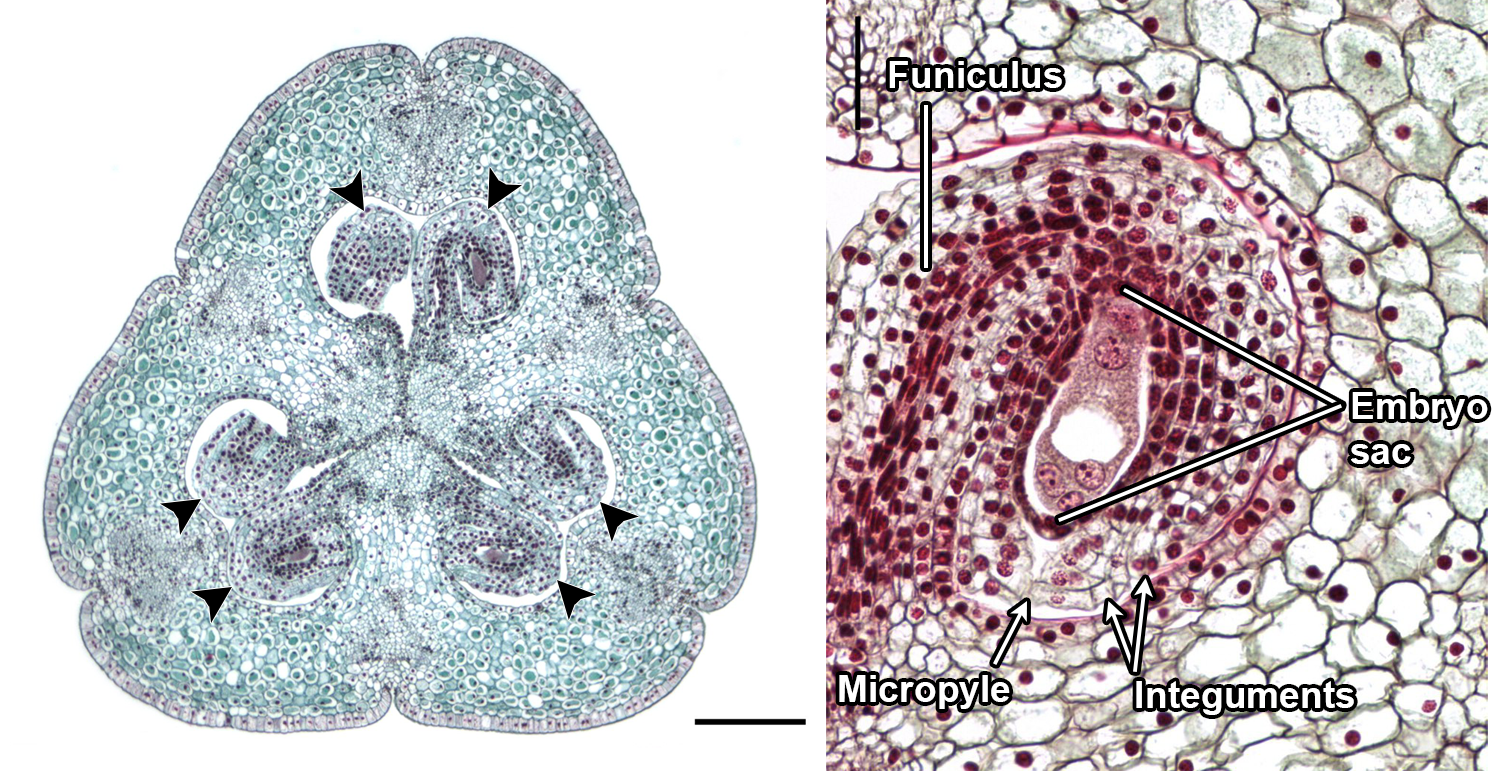
Lily ovules. Left: Cross section of the ovary of a lily (Lilium) ovary, with six ovules; the ovules are indicated by the arrowheads. Right: Longitudinal section of a single ovule with embryo sac (megagametophyte). Credit: Lilium ovary and Lilium embryo 8 nuclei (Jon Houseman & Matthew Ford, Wikimedia Commons, CC BY-SA 4.0). Images modified for DEAL.
Once the embryo sac has developed, pollination has occurred, and the pollen tube has grown into the ovary to make contact with the ovule, fertilization (fusion of egg and sperm) can occur. Typically, the pollen tube reaches the embryo sac via the micropyle (Greek, mikros + pyle = small opening), or opening, in the integuments of the ovule. There, it discharges its sperm into the embryo sac.
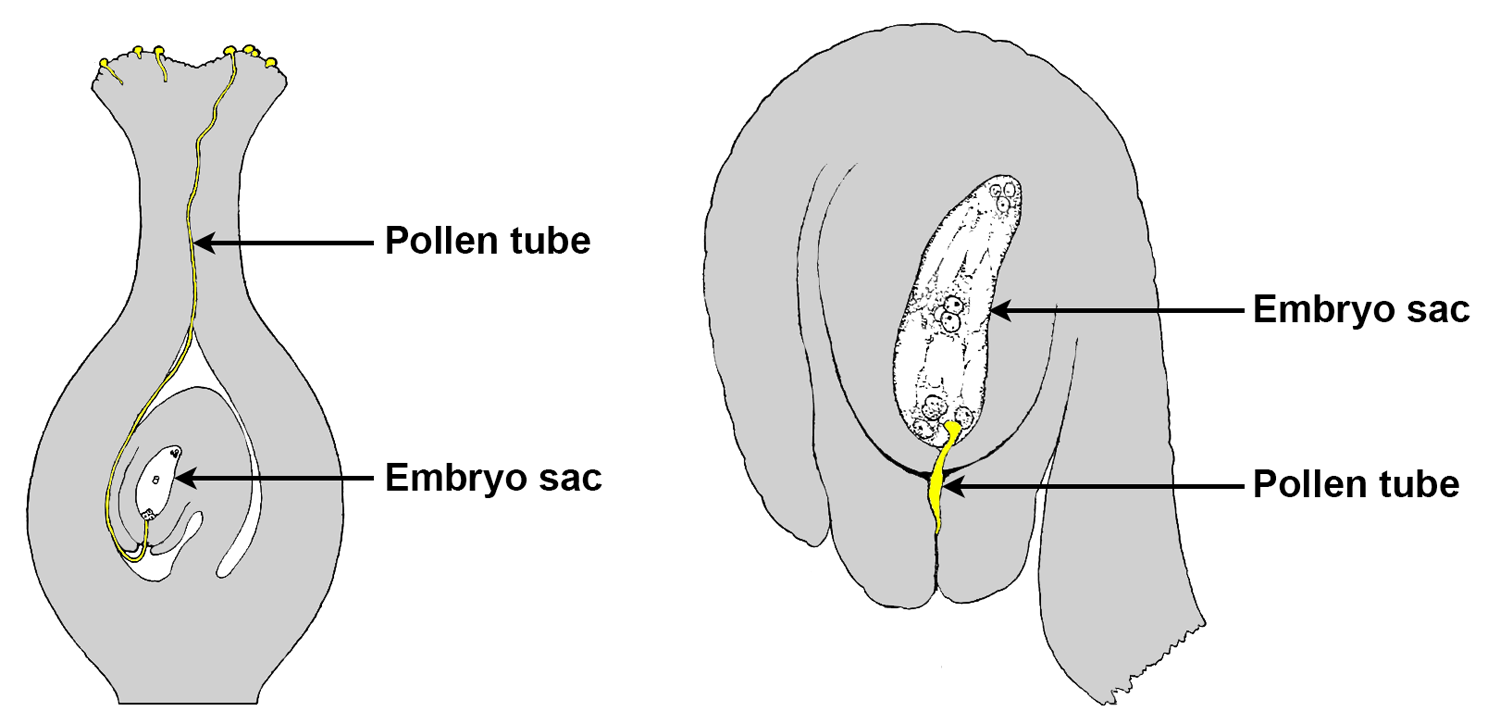
Pollen tube & fertilization. Left: Longitudinal section of a pistil, showing pollen grains on the stigma. A long pollen tube has grown out of one of the pollen grains and has made contact with the embryo sac (female gametophyte/megagametophyte) via the micropyle of the ovule. Right. Detail of an ovule, showing the pollen tube entering the ovule through the micropyle to penetrate the embryo sac at the time of fertilization. Credits: Images modified from figures 119 and 231 from Bergen & Caldwell (1914) Introduction to Botany (no known copyright restrictions).
One of the key features that distinguishes angiosperms from all other seed plants is double fertilization with endosperm formation. Double fertilization occurs in siphonogamous seed plants (i.e., seed plants with nonmotile sperm) when both sperm in a pollen tube unite with structures in a megagametophyte. In angiosperms, one sperm unites with the egg to form a diploid zygote, the first cell of a new sporophyte. The other sperm unites with the nucleus or nuclei in the large central cell of the embryo sac to form a primary endosperm nucleus. This nucleus is the first nucleus of the endosperm (Greek, endon + sperma = within seed), a type of food tissue unique to the seeds of angiosperms.
After fertilization, the ovule becomes a seed. The seed is a structure containing a young, diploid sporophyte embryo and, typically, stored food for the embryo. In angiosperms, the food in the seed may be stored in the form of endosperm, it may be stored in the cotyledon(s) (seed leaves) of the embryo, or it may be stored in both structures. The seed is protected by a seed coat, which develops from the integuments of the ovule.
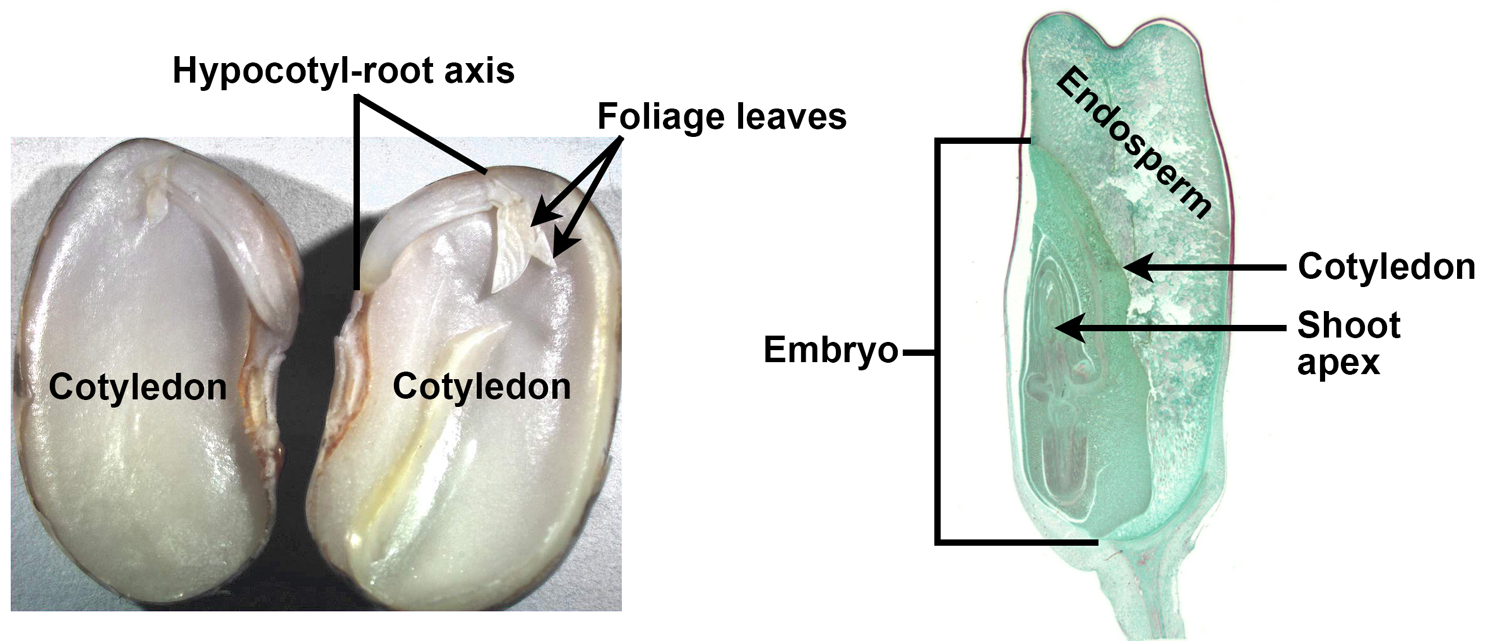
Longitudinal sections of seeds with sporophyte embryos. Left: Bean (Phaseolus) split lengthwise to show the parts of the embryo, including the two food-storing cotyledons, the hypocotyl-root axis (sporophyte embryo axis below the cotyledons), and the first foliage leaves. No endosperm is apparent. Right: Corn (Zea mays, a monocot) embryo with one cotyledon and conspicuous endosperm. Credits: Phaseolus seed (Bruce Krichoff, via flickr, CC BY 2.0); Zea kernel (Jon Houseman and Matthew Ford, Wikimedia Commons, CC BY-SA 4.0). Images modified from originals.
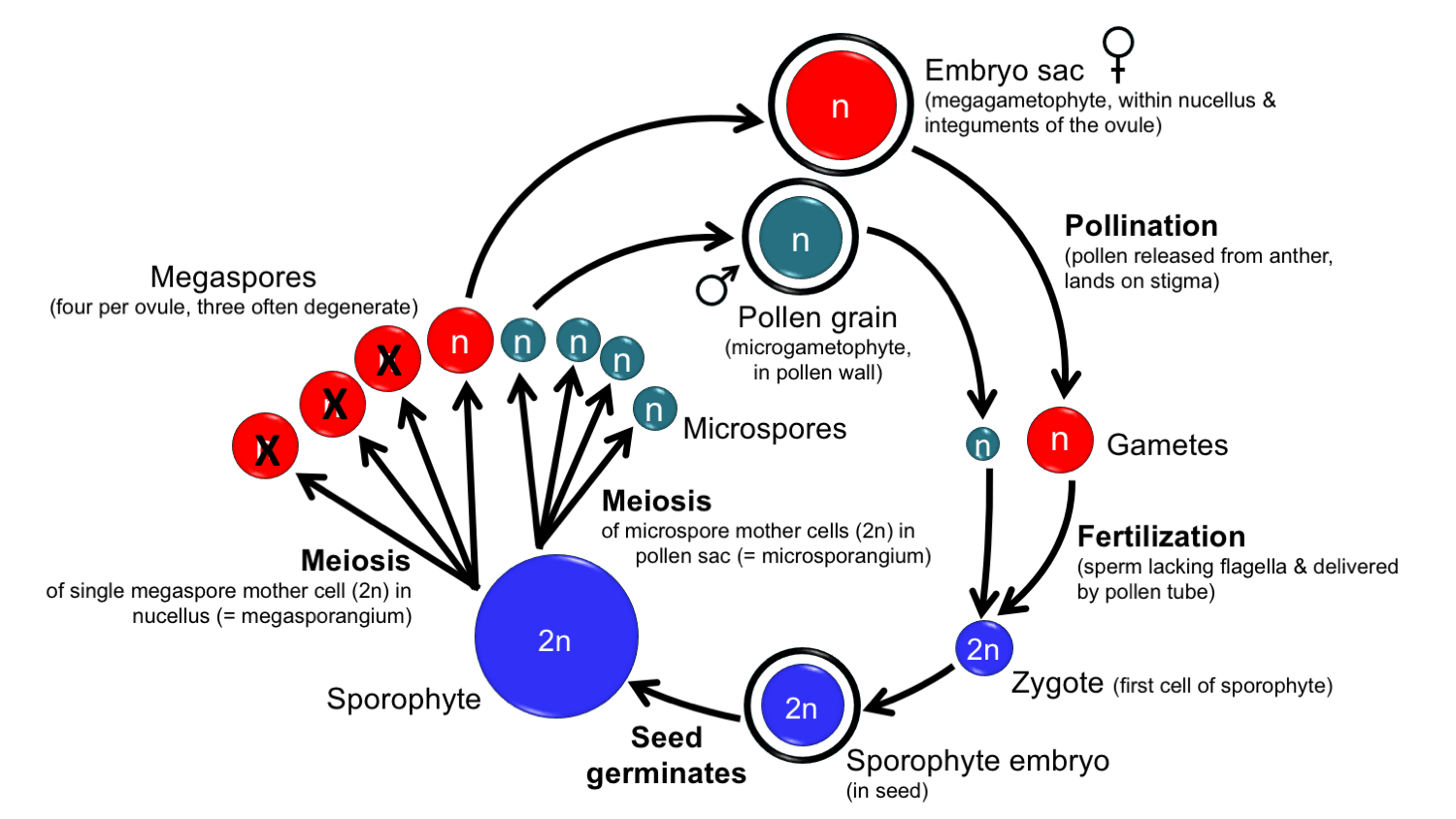
Generalized angiosperm life cycle. Summary diagram for the life cycle of an angiosperm. The diploid (2n), multicellular sporophyte bears flowers. Embryo sacs (megagametophytes) develop in the ovules, which are found in the ovary. Pollen grains (microgametophytes) develop in the pollen sacs (microsporangia) of the anther. One megaspore mother cell occurs in each ovule; it undergoes meiosis, typically giving rise to one functional megaspore. Many microspore mother cells occur in the pollen sacs; each undergoes meiosis to produce four microspores. Megaspores develop into embryo sacs, microspores into pollen grains. The pollen grains are released from the anther to land on a stigma during pollination. The pollen grain germinates, forming a pollen tube that delivers the sperm to the egg in the ovule during fertilization. The sporophyte embryo develops in the seed. Upon germination of the seed, the sporophyte resumes growth. Credit: Diagram by E.J. Hermsen (DEAL).
Pollen grain (microgametophyte) development
In flowering plants, pollen is produced in the anthers. Anthers are structures typically made up of four fused pollen sacs, or microsporangia. Microspore mother cells (also called pollen mother cells) differentiate within the pollen sacs (microsporangia) of the anthers. Each microspore mother cell undergoes meiosis to produce four haploid microspores. Each microspore then divides to produce its own pollen grain. Many pollen grains may thus be produced in a single anther.
Each microspore divides twice to produce the mature, three-celled pollen grain. The first division produces a tube cell (cell that will elongate to form the pollen tube) and a generative cell. The generative cell then divides to produce two sperm. Following pollination, the pollen grain germinates and the tube cell begins elongating to form the pollen tube.
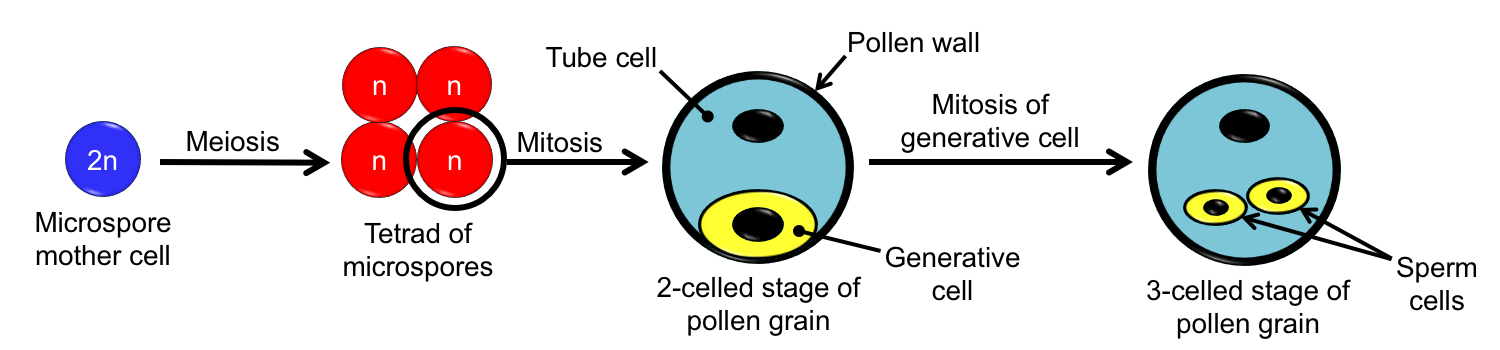
Pollen grain development. Development of the pollen grain from diploid (2n) microspore mother cell to 3-celled microgametophyte. Each micropore mother cell in a pollen sac undergoes meiosis to produce four haploid (n) microspores. Each microspore divides once to produce a 2-celled pollen grain. The generative cell divides again to yield two sperm. The tube cell will form the pollen tube that delivers the sperm following pollination. Credit: Diagram by E.J. Hermsen (DEAL), modified after Foster & Gifford (1974).
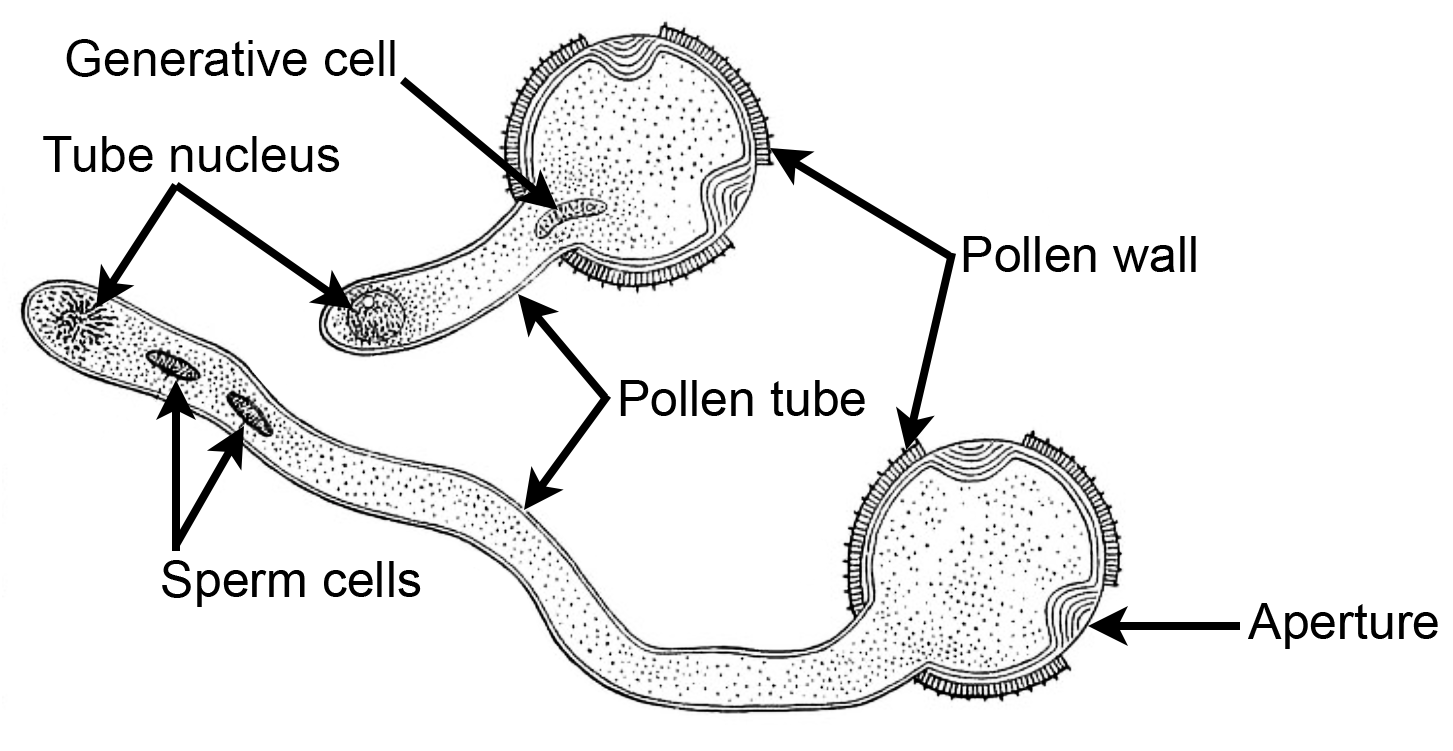
Germinated eudicot pollen grain. Two stages in the development of a germinated eudicot pollen grain. The generative cell of the two-celled stage divides to give rise to the sperm cells of the three-celled stage. Note that the pollen tube is growing through one of three apertures in the pollen wall. Depending on the plant, sperm may be formed before or after the pollen tube begins to develop. Credit: Drawing of germinated eudicot pollen grain, fig. 118 from Bergen & Caldwell (1914) Introduction to Botany (no known copyright restrictions). Image modified from original.
Embryo sac (megagametophyte) development
In angiosperms, the megagametophyte (female or egg-producing gametophyte) is also called an embryo sac. The embryo sac develops within an ovule, which is contained within the ovary of a flower. In most angiosperms, the mature embryo sac is a seven-celled, eight-nucleate structure. This type of embryo sac is also called the Polygonum-type, after the genus Polygonum (knotweed or smartweed). Most angiosperms (about 70%) are thought to have this type of embryo sac. The term "Polygonum-type" does not refer only to the final configuration of the embryo sac, but also to its sequence of development.
An important note about the diagrams in this section: The standard way to show angiosperm ovules is with the micropylar end (the end of the ovule with the micropyle or opening in the integuments) at the bottom. This choice makes sense structurally because the micropyle faces the base of the ovule in many angiosperms (an ovule orientation known as anatropous). However, when the angiosperm embryo sac and its development are shown in isolation (without the surrounding nucellus and integuments), they are often depicted with the micropyle at the top of the diagram.
This convention can be confusing when discussing embryo sac development, as it means whole-ovule and embryo-sac-only diagrams are rotated 180 degrees from each other. Thus, I have made the choice to orient the diagrams below so that the micropyle is always at the top of the diagram. Remember: No matter the orientation of the ovule or the sequence of embryo sac development, the egg cell (and associated synergids) is always at the micropylar end of the ovule.
Development of the Polygonum-type embryo sac
Embryo sac development begins with the production of megaspores. A large megaspore mother cell differentiates within the developing ovule. This megaspore mother cell undergoes meiosis to produce four haploid megaspores. One megaspore (the one furthest from the micropyle) is functional, while the rest degenerate. Thus, the Polygonum-type embryo sac exhibits monosporic (Greek, monos = one) development, meaning that it develops from a single spore.
The functional megaspore undergoes a series of free-nuclear divisions, or divisions of the nucleus without partitioning of the cytoplasm into separate cells. One nucleus divides mitotically to produce two nuclei, two nuclei divide to produce four, and four divide to produce eight.
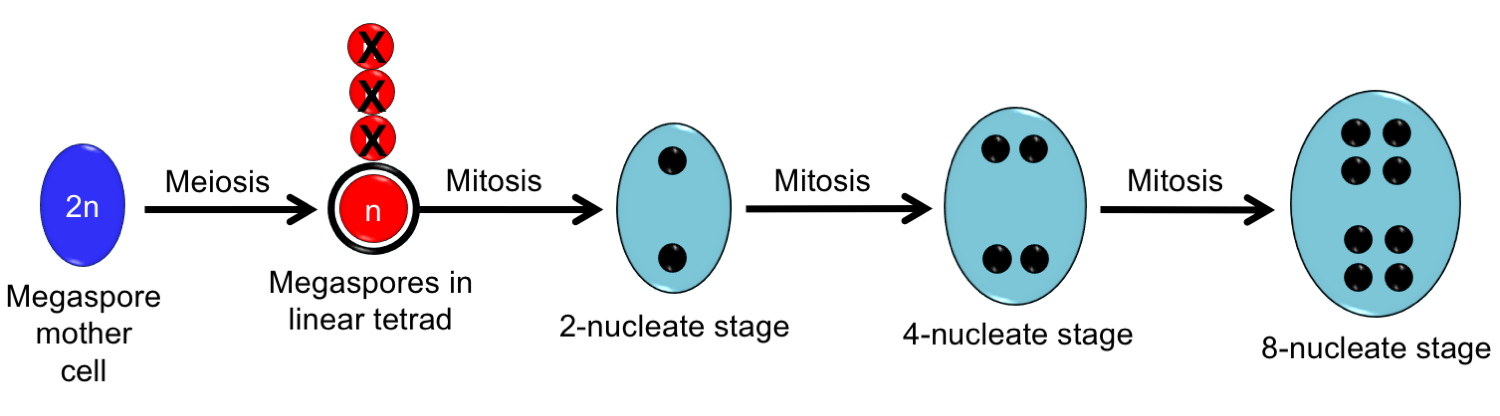
Development of the Polygonum-type embryo sac. A megaspore mother cell differentiates within the nucellus of an ovule. It undergoes meiosis to yield a tetrad (group of four) of megaspores. Three of these degenerate, and the megaspore furthest from the micropyle is functional. This megaspore undergoes free-nuclear divisions, or divisions of the nucleus without the partitioning of cells. The megaspore nucleus divides once to produce two nuclei. Each of these nuclei divides again to produce four nuclei. Each of these nuclei divides again to produce eight nuclei. The final step in development of the embryo sac will be partitioning of the cells (shown below). The diagram is oriented so that the micropyle is at the top of the figure. Credit: Diagram by E.J. Hermsen (DEAL).
Finally, the cytoplasm of the embryo sac is partitioned into seven different cells. At the end of the ovule near the micropyle are an egg cell and two synergids. At the opposite end of the embryo sac are three cells called antipodals. Finally, the large central cell contains two polar nuclei. In the Polygonum-type embryo sac, all the nuclei are haploid, or have one set of chromosomes.
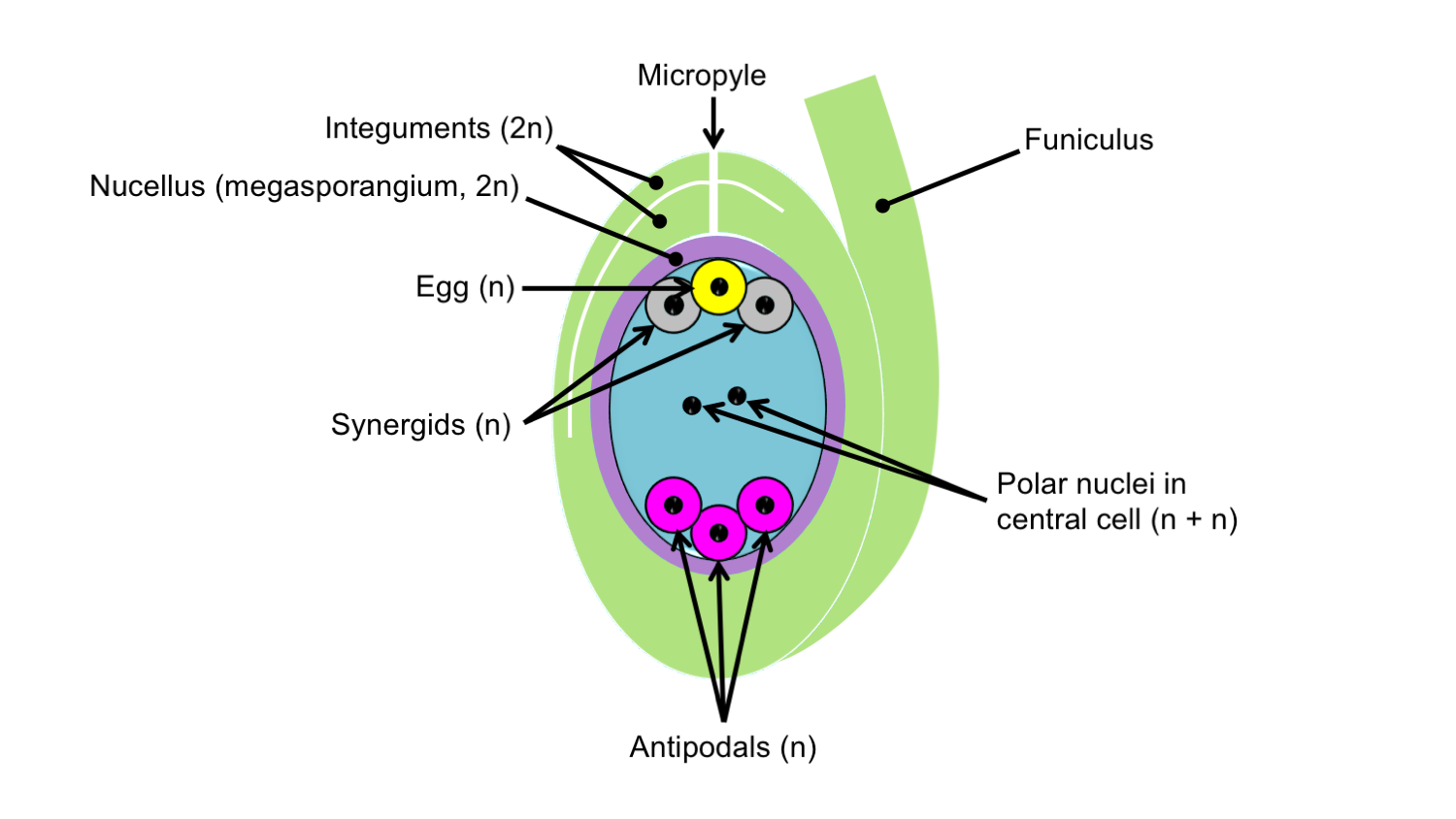
Ovule with Polygonum-type embryo sac. Idealized diagram of an ovule with a Polygonum-type embryo sac, showing the seven cells and eight nuclei surrounded by a thin nucellus (megasporangium) and double integuments. Note that the antipodals may break down and the polar nuclei may fuse to form a diploid nucleus prior to fertilization. The nucellus may also break down, so may not be observed in mature ovules. Credit: E.J. Hermsen (DEAL).
Double fertilization of the Polygonum-type embryo sac
As mentioned above, angiosperms have double fertilization. One sperm fuses with the egg to make a diploid zygote, the single cell that through division, growth, and development will eventually yield a sporophyte embryo. The other sperm fuses with the polar nuclei to form a primary endosperm nucleus. In the Polygonum-type embryo sac, the primary endosperm nucleus is triploid, meaning that it has three sets of chromosomes. One set comes from the sperm and two sets come from the polar nuclei. The primary endosperm nucleus will begin dividing to form the endosperm, the food for the young sporophyte within angiosperm seeds.
What happens to the other cells and structures of the ovule: the antipodals, synergids, nucellus, and integuments? The antipodals degenerate, either prior to or following fertilization. They have no clear function in the embryo sac. The synergids are thought to play a role in fertilization, although these also degenerate. Typically, the nucellus degenerates prior to maturation of the seed. The integument or integuments become the seed coat.
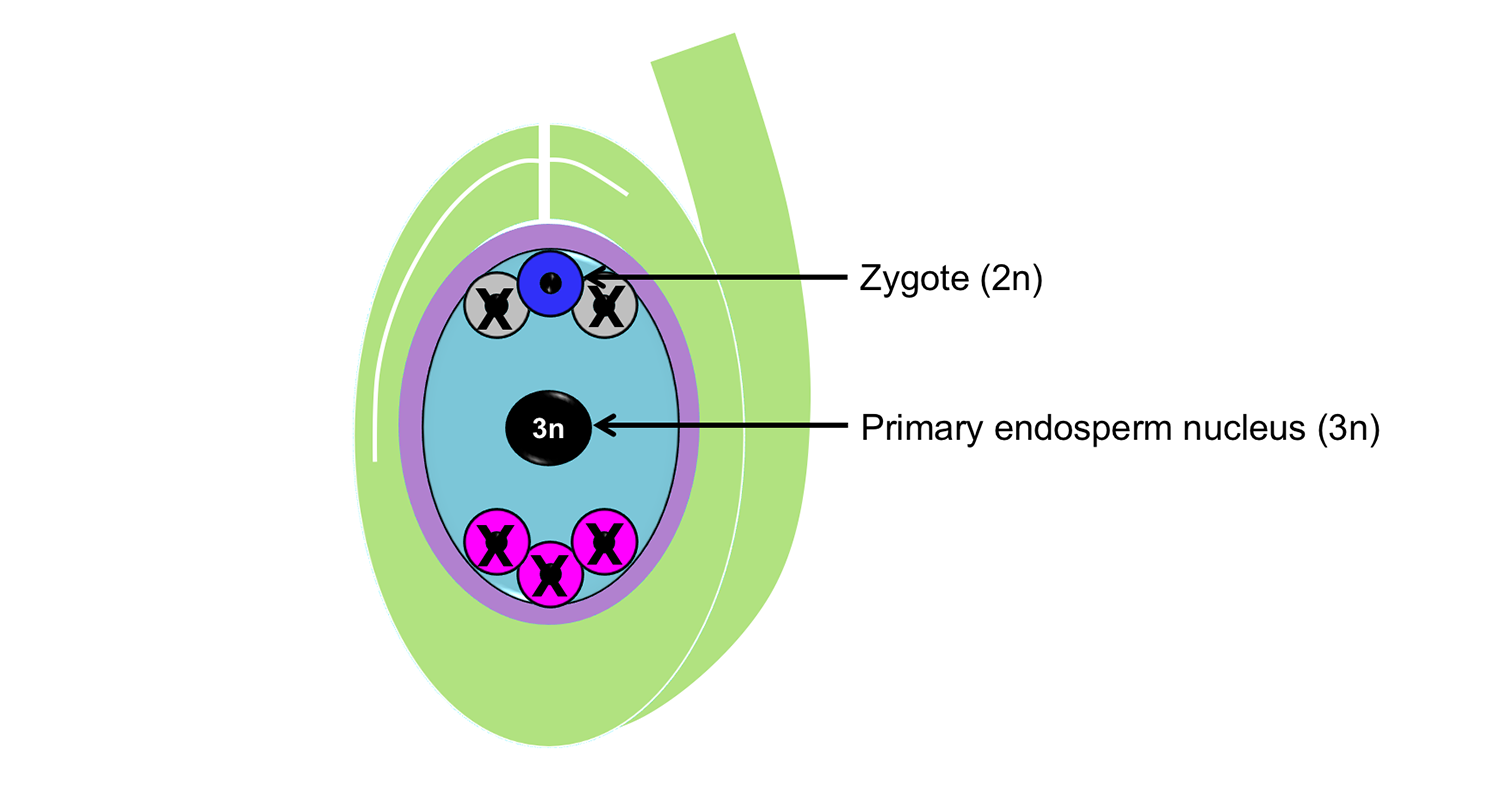
Polygonum-type embryo sac after double fertilization. Idealized diagram showing the Polygonum-type embryo sac following double fertilization. During double fertilization, the egg and a sperm unite to form a diploid zygote. The other sperm unites with the polar nuclei to form a triploid primary endosperm nucleus. The antipodals degenerate. The synergids play a role in fertilization, but also degenerate. Credit: Diagram by E.J. Hermsen (DEAL).
Evolution of the embryo sac
Although the Polygonum-type embryo sac is the most common type of embryo sac in angiosperms, it is not thought to be the ancestral type. Rather, studies on Amborella and other basal angiosperms in the Austrobaileyales and Nymphaeales have shed light on the origin of the Polygonum-type embryo sac and the evolution of other types of embryo sacs in angiosperms.
Ancestral organization of the angiosperm embryo sac
The Nuphar/Schisandra-type of embryo sac is thought to be the ancestral type of embryo sac for crown-group angiosperms, or all living angiosperms and their most recent common ancestor (see here, here, here, and here). This type of embryo sac is found in members of Austrobaileyales and Nymphaeales, two orders of basal or ANA-grade angiosperms. Nuphar (Nymphaeales) is a type of water lily and Schisandra (Austrobaileyales) is a type of shrub native to the southeastern United States, Mexico, and eastern to southeastern Asia.

Nuphar and Schisandra flowers. Left: Yellow pond-lily (Nuphar lutea, Nymphaeales). Right: Bay starvine (Schisandra glabra, Austrobaileyales). Credits: Yellow water-lily (Randi Hausken, via Wikimedia Commons, CC BY-SA 2.0); Schisandra glabra (Helen Lowe Metzman/USGS Bee Inventory and Monitoring, via flickr, Public Domain). Images modified from originals.
The Nuphar/Schisandra-type embryo sac begins development in a manner similar to the Polygonum-type embryo sac. It develops from a single megaspore that undergoes two sets of free-nuclear divisions, to produce a 4-nucleate stage. Cellular partitioning then results in a 4-celled, 4-nucleate embryo sac. The embryo sac has an egg, two synergids, and a polar nucleus in a central cell. No antipodals are formed. Double fertilization in this type of embryo sac yields a diploid zygote and a diploid primary endosperm nucleus (one set of chromosomes from a sperm + one set from the polar nucleus = two sets of chromosomes in the primary endosperm nucleus).
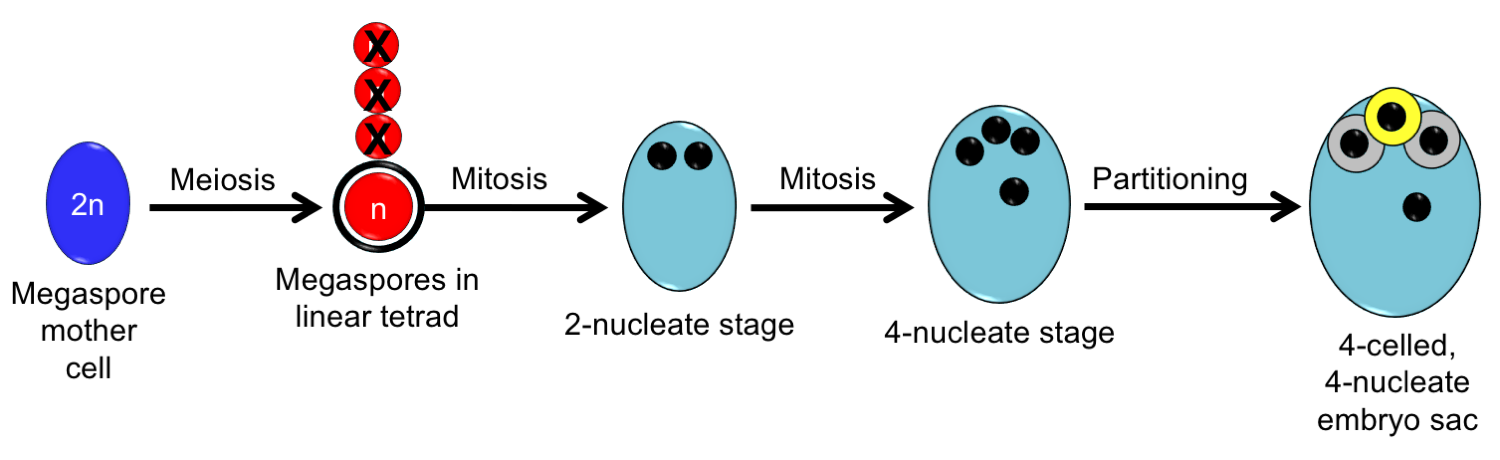
The Nuphar/Schisandra-type embryo sac. Development of the Nuphar/Schisandra-type embryo sac is similar to development of the Polygonum-type embryo sac. Differences include that nuclei are located only at the micropylar end and partitioning of cells happens after the 4-nucleate stage. The mature embryo sac has an egg cell, two synergids, and a single polar nucleus. Credit: Diagram by E.J. Hermsen (DEAL), modified after Friedman & Ryerson (2009) and other papers on megagametogenesis in ANA-grade angiosperms (see references).
Modularity of the embryo sac
Research on development of the megagametophyte in ANA-grade angiosperms suggests that the four cells of the Nuphar/Schisandra-type embryo sac may act as a module or basic building block upon which other types of angiosperm embryo sacs are based (see here, here, here, and here). Thus, the Polygonum-type embryo sac may have evolved from the Nuphar/Schisandra-type via a duplication of the basic 4-celled, 4-nucleate unit of egg cell + 2 synergids + 1 polar nucleus in the central cell. The antipodals plus one of the polar nuclei in the central cell of the Polygonum-type embryo sac can thus be viewed simply as a doubling of this basic unit at the opposite end of the embryo sac.
Interestingly, Amborella, the basalmost living angiosperm, does not have a four-celled, four-nucleate embryo sac. Rather, it has an eight-celled, nine-nucleate embryo sac, which looks like a Polygonum-type embryo sac with one additional synergid. It is thought that the Amborella-type embryo sac evolved independently from the Nuphar/Schisandra-type embryo sac and not from the Polygonum-type embryo sac (see here).
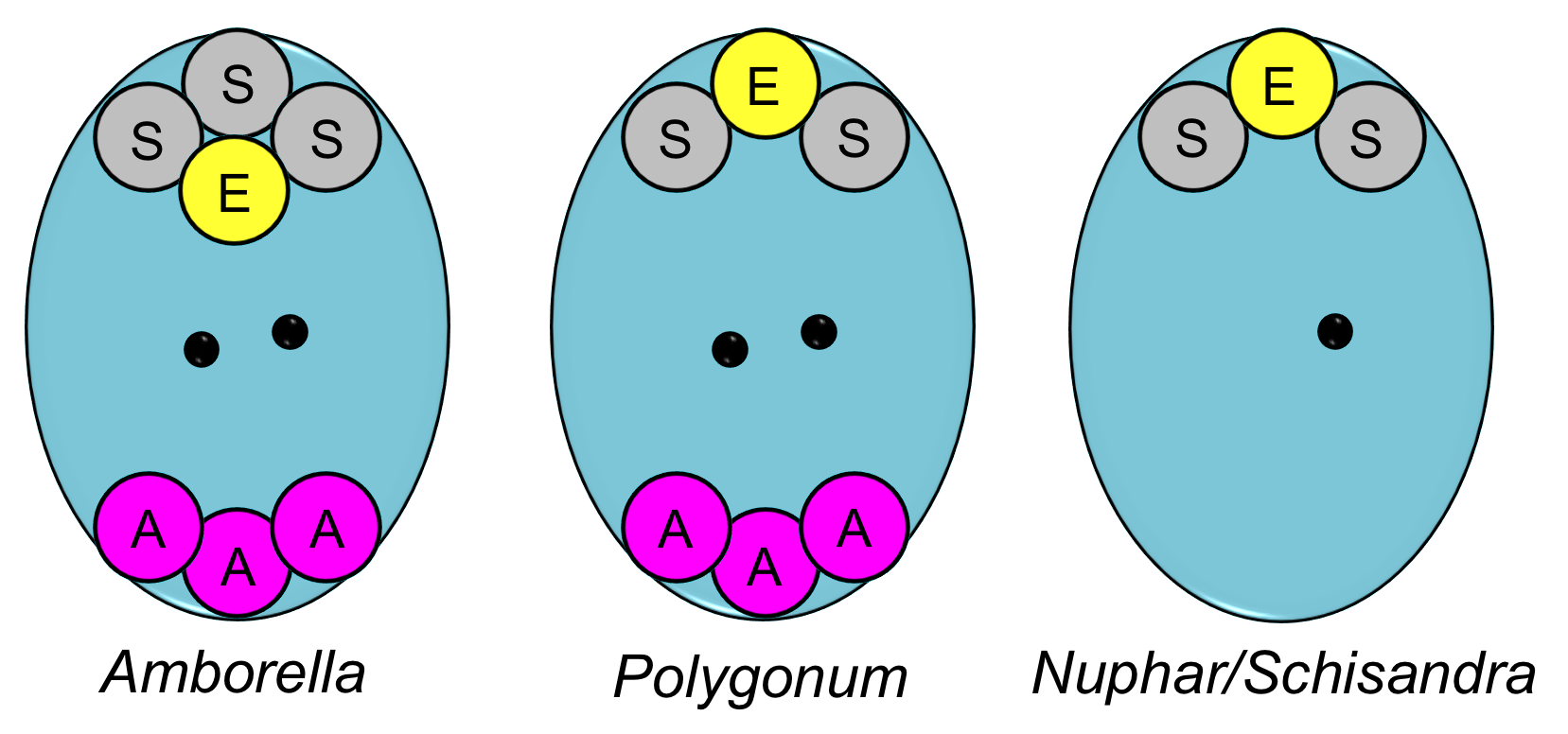
Comparison of embryo sacs. Left: Amborella-type embryo sac with an egg (E), three synergids (S), three antipodals (A), and two polar nuclei (black dots). Center: Polygonum-type embryo sac with an egg, two synergids, three antipodals, and two polar nuclei. Right: Nuphar/Schisandra-type with an egg, two synergids, and one polar nucleus. All embryo sacs are oriented with the micropylar end up. Credit: Diagram by E.J. Hermsen (DEAL), modified after Friedman & Ryerson (2009) and other papers on megagametogenesis in ANA-grade angiosperms (see references).
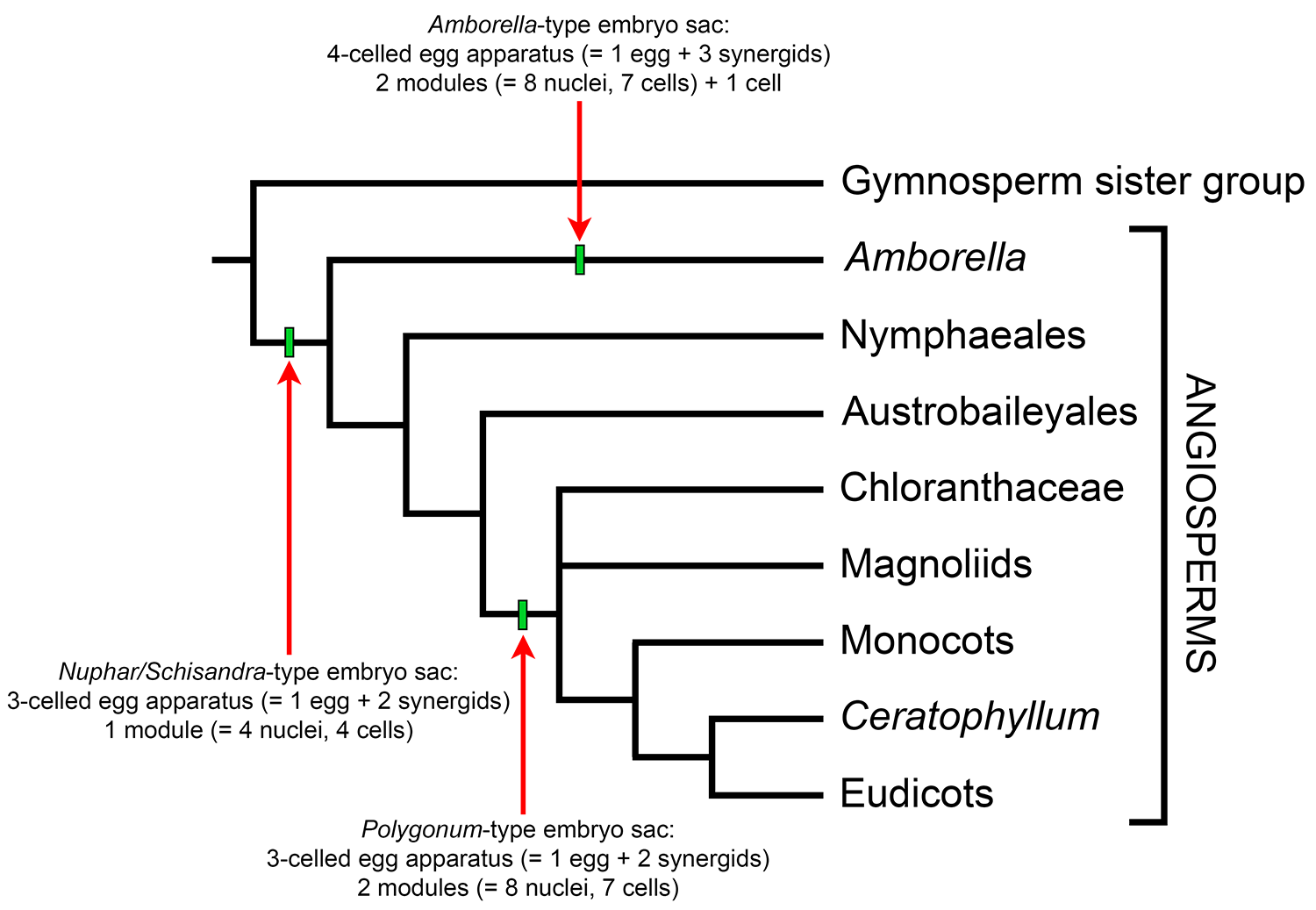
Hypothesized evolution of embryo sacs mapped unto a phylogeny. Evolution of angiosperm embryo sacs under the modular theory, as mapped unto a simplified tree of angiosperm relationships. The Nuphar/Schisandra-type (4-celled, 4-nucleate) is a synapomorphy for crown-group angiosperms. The Amborella-type (8-celled, 9-nucleate) evolved only in the Amborella lineage. The Polygonum-type (7-celled, 8-nucleate) is a synapomorphy for the clade including all living angiosperms above the ANA-grade (i.e., Amborella, Nymphaeales, and Austrobaileyales). Credit: Diagram by E.J. Hermsen (DEAL), modified after Friedman & Ryerson (2009) and other papers on megagametogenesis in ANA-grade angiosperms (see references).
Other embryo sacs
In addition to Polygonum-, Nuphar/Schisandra-, and Amborella-type embryo sacs, other types of embryo sacs have evolved in other groups of angiosperms. We will not review them all here. In a general sense, angiosperm embryo sacs fall into one of the following categories:
- Monosporic: In this type of development, the embryo sac arises from one megaspore, and the other three megaspores degenerate. Examples: The three types of embryo sacs detailed above (Polygonum, Nuphar/Schisandra, and Amborella) are monosporic.
- Bisporic: In this type of development, a single embryo sac arises from two of the four megaspore nuclei produced by meiosis of the megaspore mother cell. The other two nuclei degenerate.
- Tetrasporic: In this type of development, all four nuclei produced by meiosis of a single megaspore mother cell give rise to a single embryo sac. Tetrasporic embryo sacs may show the most variation in development and final structure, and the endosperm cells may have more than three sets of chromosomes. One species with a tetrasporic embryo sac, Peperomia hispidula, forms a 15n primary endosperm nucleus, or a nucleus with 15 sets of chromosomes (read the study here)!
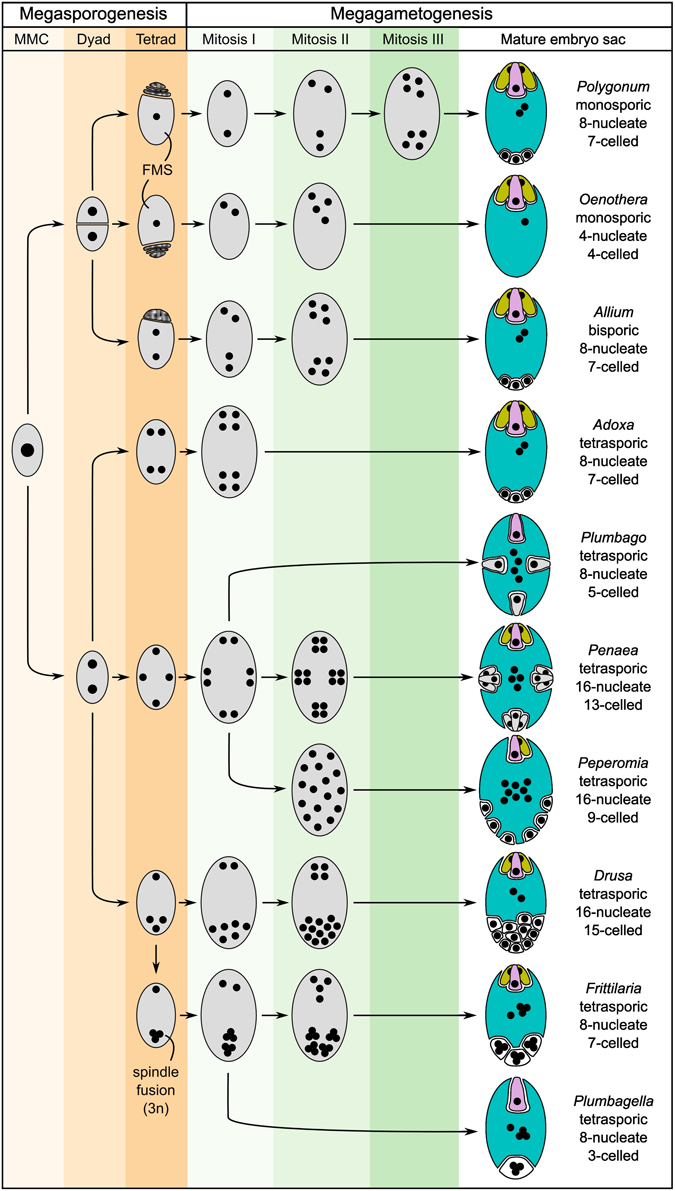
Some variations in angiosperm embryo sac development. Diagram (after Maheshwari 1950) showing major variations in the development of the embryo sac (megagametophyte or female gametophyte) of angiosperms. The micropyle is a the top of the image in all diagrams. Megasporogenesis is the process that produces a tetrad (group of four) megaspores from the megaspore mother cell (MMC) through division by meiosis. FMS = functional megaspore. Megagametogenesis is the development of the female gametophyte (embryo sac) through mitotic divisions of the nuclei, cell partitioning, and differentiation (specialization) of cells. In the mature embryo sac, the egg cell is the large pink cell at the apex; the synergids are the greenish cells associated with the egg. Credit: Figure 2 from Schmid et al. (2015) Frontiers in Plant Science (CC BY 4.0).
Selected references & addition reading
Note: Free full text is made available by the publisher for items marked with a green asterisk.
Journal articles
* Friedman, W.E., and K.C. Ryerson. 2009. Reconstructing the ancestral female gametophyte of angiosperms: insights from Amborella and other ancient lineages of flowering plants. American Journal of Botany 96: 129-143. https://doi.org/10.3732/ajb.0800311
* Friedman, W.E., and J.H. Williams. 2003. Modularity of the angiosperm female gametophyte and its bearing on the early evolution of endosperm in flowering plants. Evolution 57: 216-230. https://doi.org/10.1111/j.0014-3820.2003.tb00257.x
* Friedman, W.E., and J.H. Williams. 2004. Developmental evolution of the sexual process in ancient flowering plant lineages. The Plant Cell 16: S119-S132. https://doi.org/10.1105/tpc.017277
Friedman, W.E., W.N. Gallup, and J.H. Williams. 2003. Female gametophyte development in Kadsura: implications for Schisandraceae, Austrobaileyales, and the early evolution of flowering plants. International Journal of Plant Sciences 164: S293-S305. https://doi.org/10.1086/376877
* Madrid, E.N., and W.E. Friedman. 2009. The developmental basis of an evolutionary diversification of female gametophyte structure in Piper and Piperaceae. Annals of Botany 103: 869-884. https://doi.org/10.1093/aob/mcp011
* Madrid, E.N., and W.E. Friedman. 2010. Female gametophyte and early seed development in Peperomia (Piperaceae). American Journal of Botany 97: 1-14. https://doi.org/10.3732/ajb.0800423
* Rudall, P.J., M.V. Remizowa, A.S. Beer, E. Bradshaw, D.W. Stevenson, T.D. MacFarlane, R.E. Tuckett, S.R. Yadav, and D.D. Sokoloff. 2008. Comparative ovule and megagametophyte development in Hydatellaceae and water lilies reveal a mosaic of features among early angiosperms. Annals of Botany 101: 941-956. https://doi.org/10.1093/aob/mcn032
* Schmid, M.W., A. Schmidt, U. Grossniklaus. 2015. The female gametophyte: an emerging model for cell type-specific systems biology in plant development. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2015.00907
Tobe, H., Y. Kimoto, and N. Prakash. 2007. Development and structure of the female gametophyte in Austrobaileya scandens (Austrobaileyaceae). Journal of Plant Research 120: 431-436. https://doi.org/10.1007/s10265-007-0085-0
Williams, J.H., and W.E. Friedman. 2002. Identification of diploid endosperm in an early angiosperm lineage. Nature 415: 522-526. https://doi.org/10.1038/415522a
* Williams, J.H., and W.E. Friedman. 2004.The four-celled female gametophyte of Illicium (Illiciaceae; Austrobaileyales): implications for understanding the origin and early evolution of monocots, eumagnoliids, and eudicots. American Journal of Botany 91: 332-351. https://doi.org/10.3732/ajb.91.3.332
Books & textbooks
Bergen, J.Y., and O.W. Caldwell. 1914. Introduction to Botany. Ginn and Company, Boston. Read online at the Internet Archive.
Esau, K. 1977. Anatomy of seed plants, 2nd ed. John Wiley & Sons, New York.
Evert R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York.
Foster, A.S., and E.M. Gifford. 1974. Comparative Morphology of Vascular Plants, 2nd ed. W.H. Freeman and Co., San Francisco.
Maheshwari, P. 1950. An Introduction to the Embryology of Angiosperms. McGraw-Hill Book Company, Inc., New York, Toronto, London. Read online at the Internet Archive.
Simpson, M.G. 2010. Plant Systematics, 2nd ed. Academic Press, Burlington, Massachusetts.
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
First released 9 August 2019; last updated 11 June 2020.