Section contents
Angiosperms (flowering plants)
- Flowers
- Life cycle
- Pollination
- Fruits
- Fruit & seed dispersal
- Leaf architecture ←
- Overview of angiosperm phylogeny
Related pages/background reading
Feature image: Leaf of witch hazel (Hamamelis) showing reticulate venation. Credit: E.J. Hermsen (DEAL).
Introduction
Angiosperm leaves are perhaps the most common and important macrofossils in the angiosperm paleobotanical record. Leaves not only provide information about fossil angiosperm diversity and affinities, but they can also be used in other types of analyses, like estimating paleoclimatic parameters. Nevertheless, leaf architecture (leaf morphology and venation patterns) is not often documented in detail in descriptions of modern plants. Leaves are often described based on their phyllotaxy (arrangement on the stem), whether they are simple or compound, size, major venation pattern (e.g., pinnate or palmate, type of lateral veins), type of margin (edge of the leaf blade), and type of indumentum (type and density of hairs, if present). Other unusual features, like stipules (paired structures at the base of the petiole where the leaf attaches to the stem), nectaries, etc., may also be noted. However, the finer details of the venation are often not described and many other characteristics may be left out.
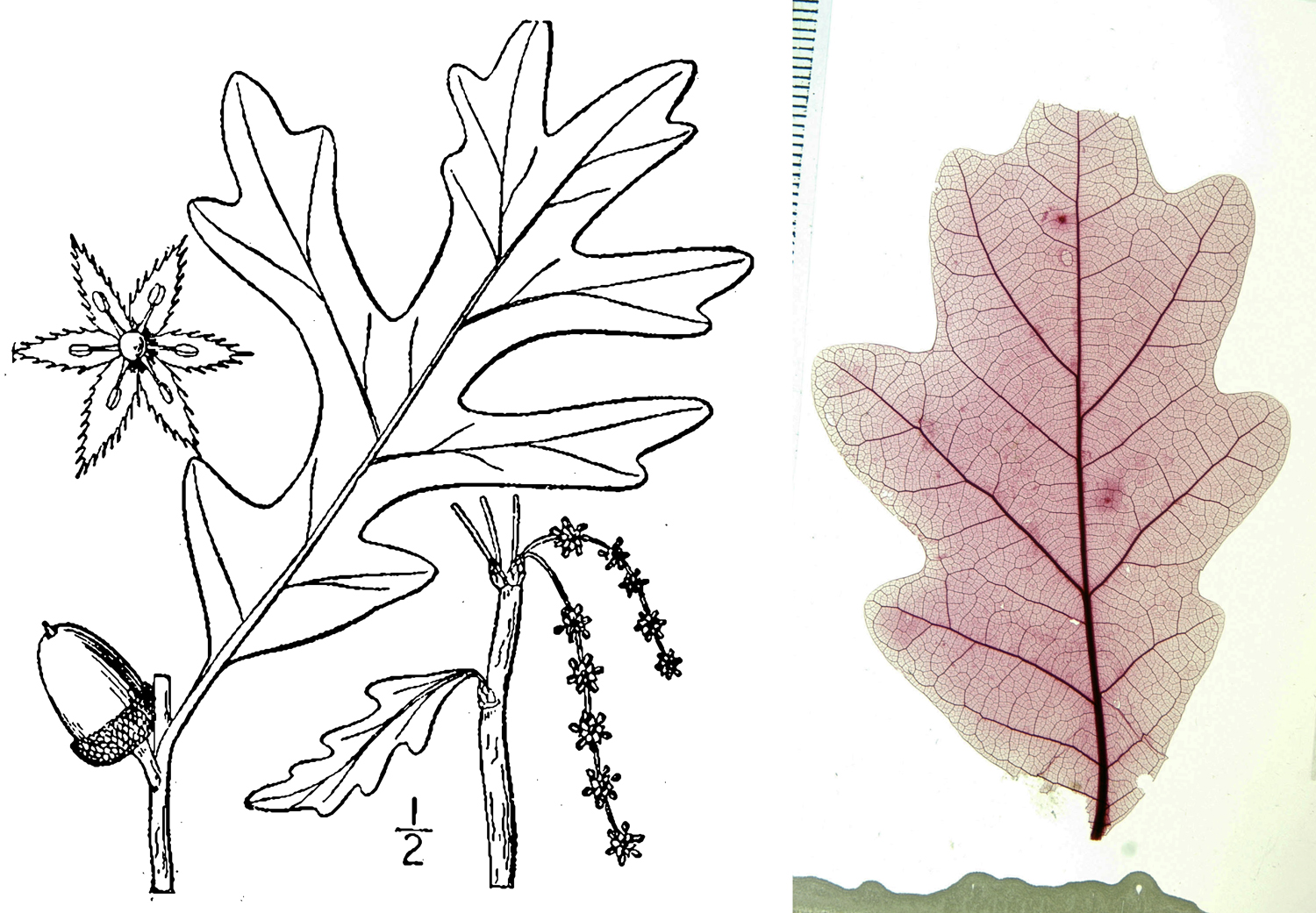
There are a few reasons that certain details of leaf architecture tend to be overlooked in descriptions of modern plants. For living plants, botanists often have access to the whole plant, or at least a specimen with more than one organ. The purpose of most plant descriptions is not to provide a catalogue of every possible observable feature on a plant, but rather to provide a summary of the characteristic features that can readily be noted, particularly in the field. Describing leaf architecture is time consuming and may not add many generally useful differentiating characters to a whole-plant description. Thus, many studies and classifications of leaf architecture grow out of the work of angiosperm paleobotanists, who have no other choice but than to try to identify plants based on their leaves, which are often preserved as isolated organs in the fossil record.
In order to study the architecture of modern leaves for comparison to fossil leaves, paleobotanists may visit herbaria (collections of dried, pressed plants) to examine the leaves of modern plants. They may also make leaf clearings. A leaf clearing is a leaf that has been processed using chemicals to remove the pigments; afterwards, it is stained. Staining the leaf brings out the venation pattern, since the vascular tissues appear darker than the surrounding mesophyll when stained. Stained leaves are then mounted, or placed in a medium—a natural resin like Canada balsam (a conifer resin), a synthetic resin, an oil like cedar oil, or another substance—on a large glass slide or between transparent plastic sheets in order to preserve them and keep them from drying out. Clearing, staining, and mounting leaves allows them to be studied, photographed, and stored for long periods of time.

Simple vs. compound leaves
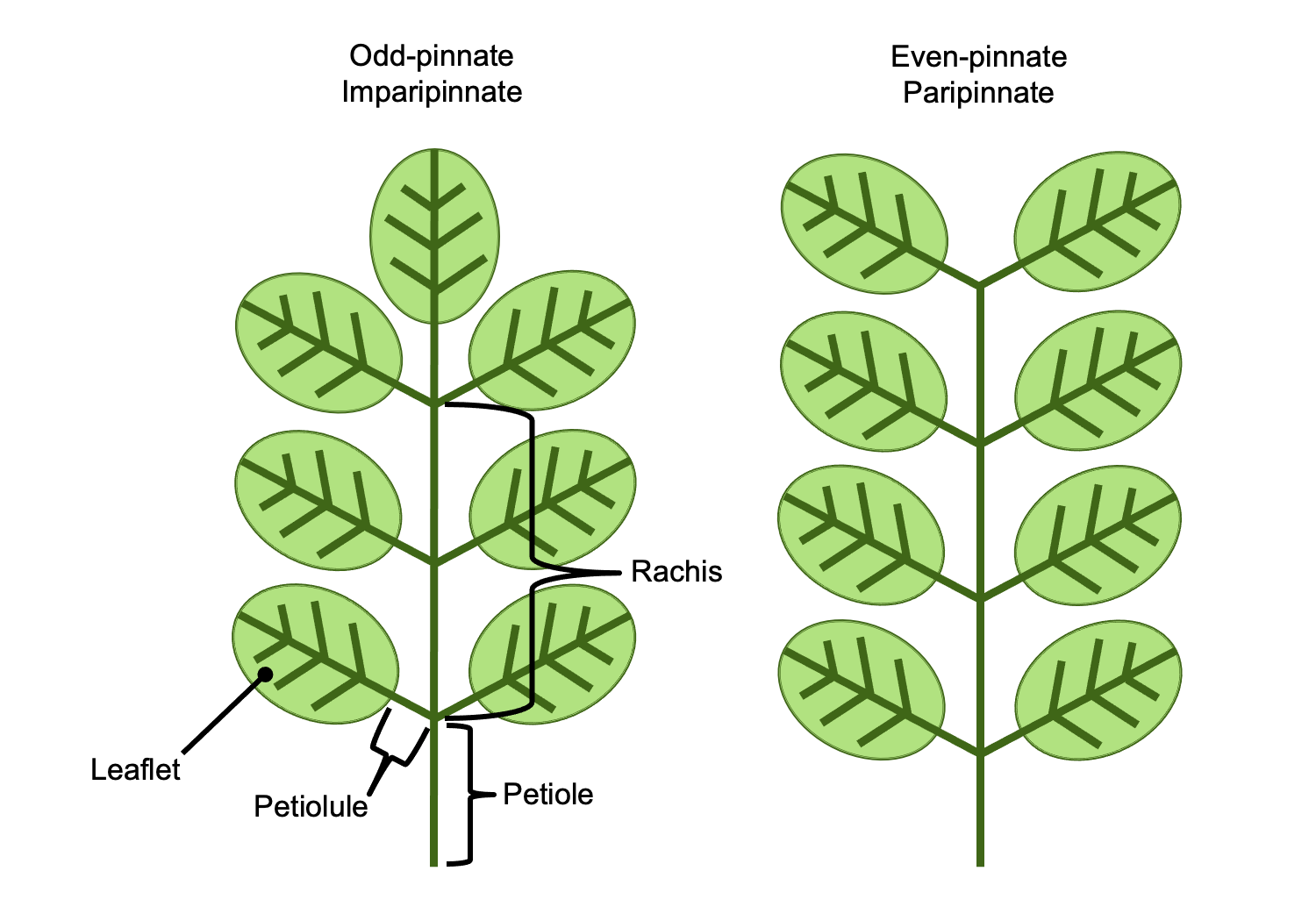
Simple leaves are leaves in which the leaf blade (lamina) is not divided. Compound leaves are leaves that are divided into leaflets that resemble simple leaves (to read more and view diagrams of simple and compound leaves, visit the DEAL Leaf Structure & Evolution page). Determining whether a leaf is simple or compound is one of the most fundamental and (theoretically) straightforward steps in describing an angiosperm leaf. In living plants, confusion is most likely to occur in the case of pinnately compound leaves. A pinnately compound leaf has a rachis (central axis) bearing lateral leaflets. A pinnately compound leaf may thus look similar to a branch bearing simple leaves.
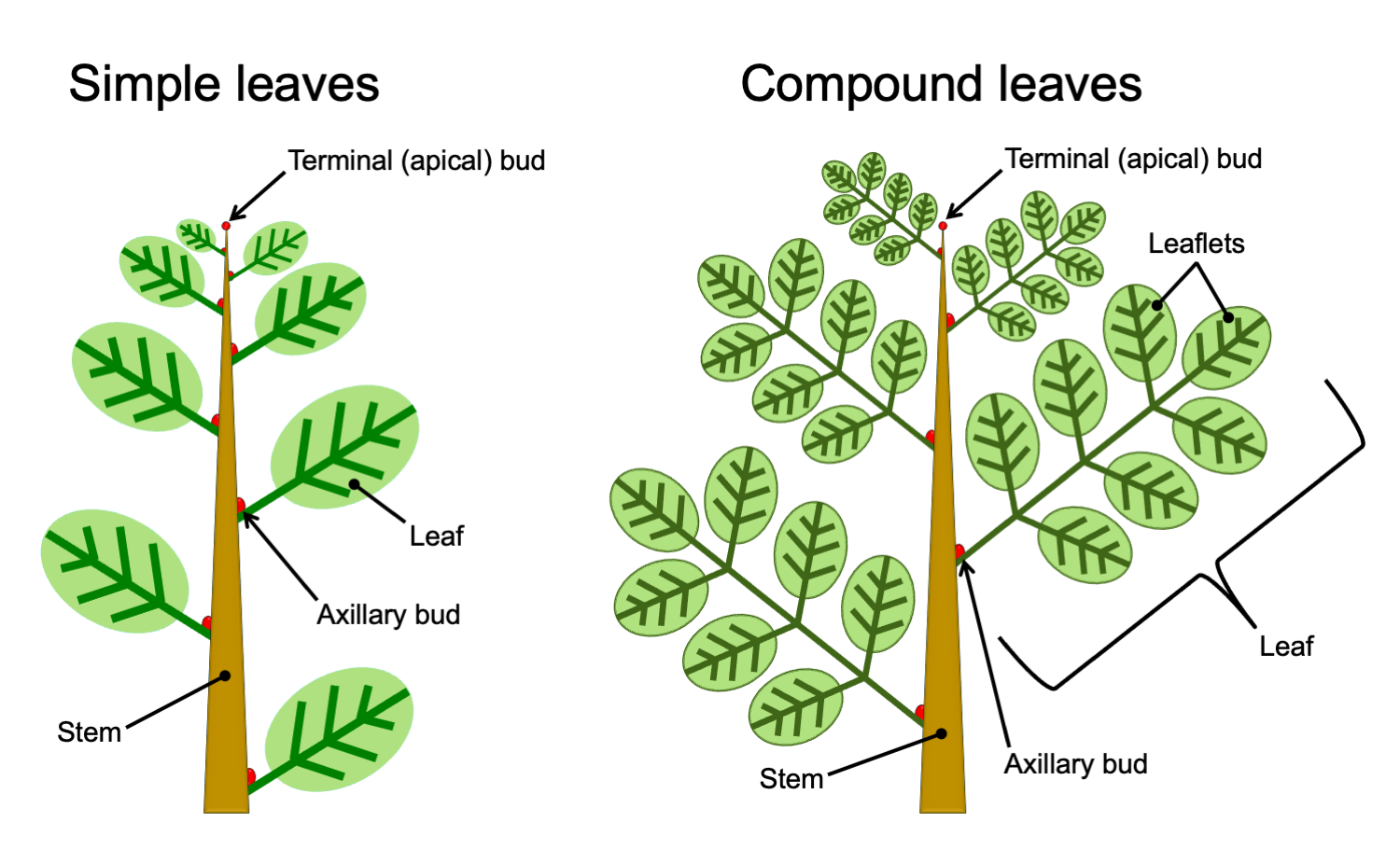
Usually, the difference between a leaf and a branch is obvious in living plants. Stems are often woody and may have leaves arranged in a spiral or several rows, whereas leaves are not woody and often have leaflets in only two rows. Furthermore, the whole leaf structure is relatively flat (planar), which is often not the case for leafy stems. When the difference between a branch bearing leaves and a compound leaf is not clear, looking for the buds can help:
- Axillary buds: Angiosperms have axillary branching, where branches develop from buds that form in the axils of the leaves. An axil is the angle formed between the upper side of the leaf and the stem to which it is attached (read more about it on the DEAL Branching page). In a compound leaf, a bud will occur where the leaf attaches to the stem, but buds will not occur where the leaflets attach to the rachis.
- Terminal (apical) bud: In angiosperms, a stem elongates from a terminal bud, which is a growing point (apical meristem and associated structures) at the stem tip. This terminal bud may be protected by bud scales or by a series of young, developing foliage leaves. Compound leaves lack a terminal bud. Pinnately compound leaves of angiosperms may end in one of two ways:
-
- Odd-pinnate or imparipinnate leaves have a terminal leaflet.
- Even-pinnate or paripinnate leaves have no terminal leaflet, and the end of the rachis truncates with a pair of leaflets.


Unfortunately, things are not so simple for paleobotanists. It may be difficult to tell whether a fossil angiosperm leaf is simple or compound because many of the above clues may be missing in fossil leaves. Many fossil structures are flattened when they are preserved. Fossil compound leaves are often not preserved as complete structures; instead, leaflets may be preserved in isolation. Buds—either axillary or terminal—are also typically not preserved, because fossil angiosperm leaves are rarely found attached to branches.
Paleobotanists may thus have to rely on luck (for example, preservation of a branch with leaves or of a complete compound leaf) or careful comparison to leaves on related modern plants to determine whether a fossilized leaf-like structure is more likely to be a simple leaf or a leaflet from a compound leaf.


Important terminology note!
The venation and margin terms described below on this page can be applied to simple angiosperm leaves or to the leaflets of compound angiosperm leaves. Only the word "leaf" is used in defining terms below for the sake of simplicity, but the word "leaflet" can be substituted when applicable.
Reticulate venation patterns
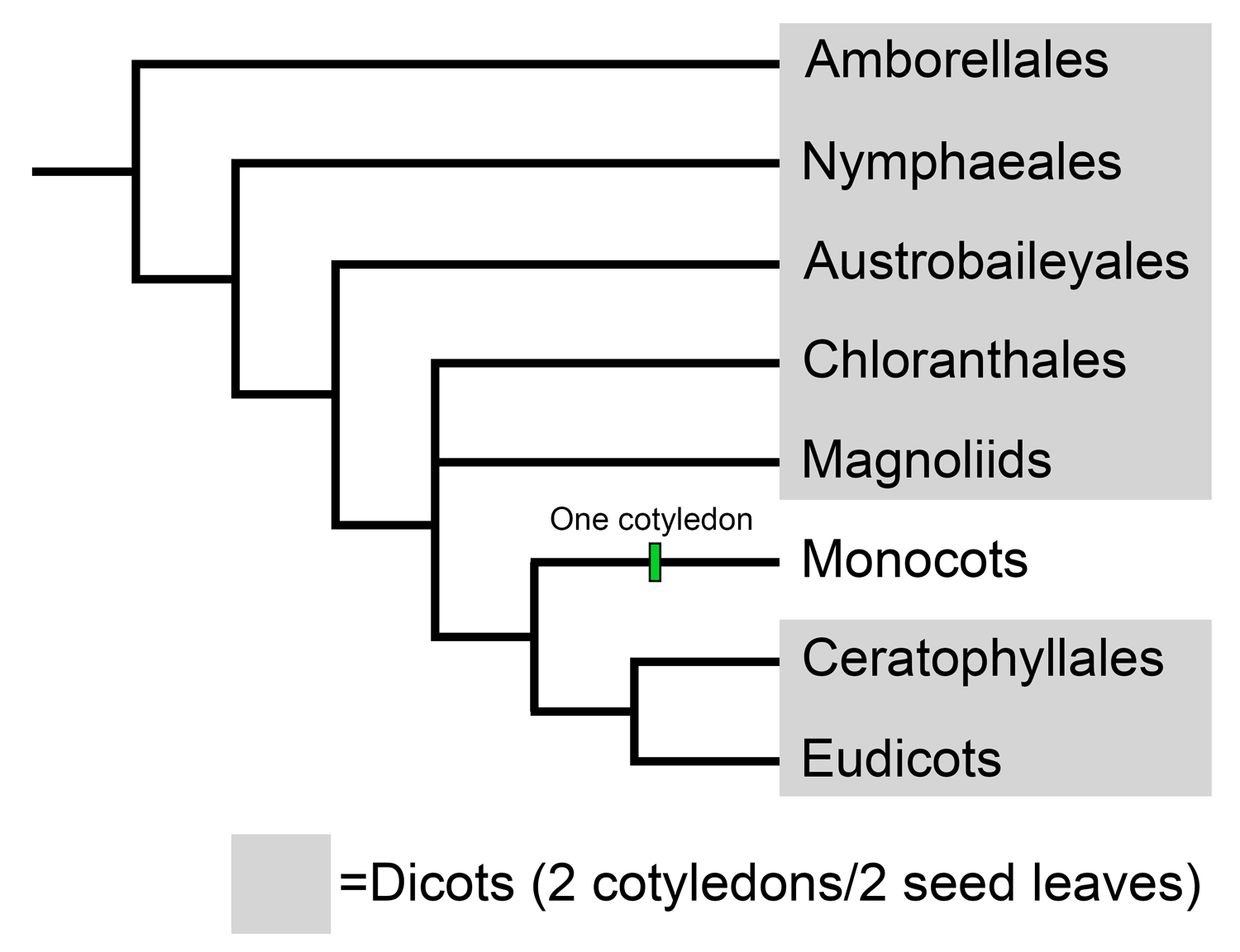
Complex schemes have been developed to characterize and determine the relationships of fossil leaves with reticulate venation on the basis of their architecture—in other words, structure, or features like shape, venation, and other characteristics. Most reticulately veined leaves are leaves of dicots. "Dicot" is a historical category in angiosperm classification. A dicot is an angiosperm that has an embryo with two cotyledons, or seed leaves. Today, dicots are recognized as being a paraphyletic group that includes plants found in the basal angiosperms, Chloranthales, magnoliids, Ceratophyllales, and eudicots. Thus, dicots are essentially angiosperms that are not monocots. (For more on angiosperm classification, see the overview of angiosperm phylogeny.)

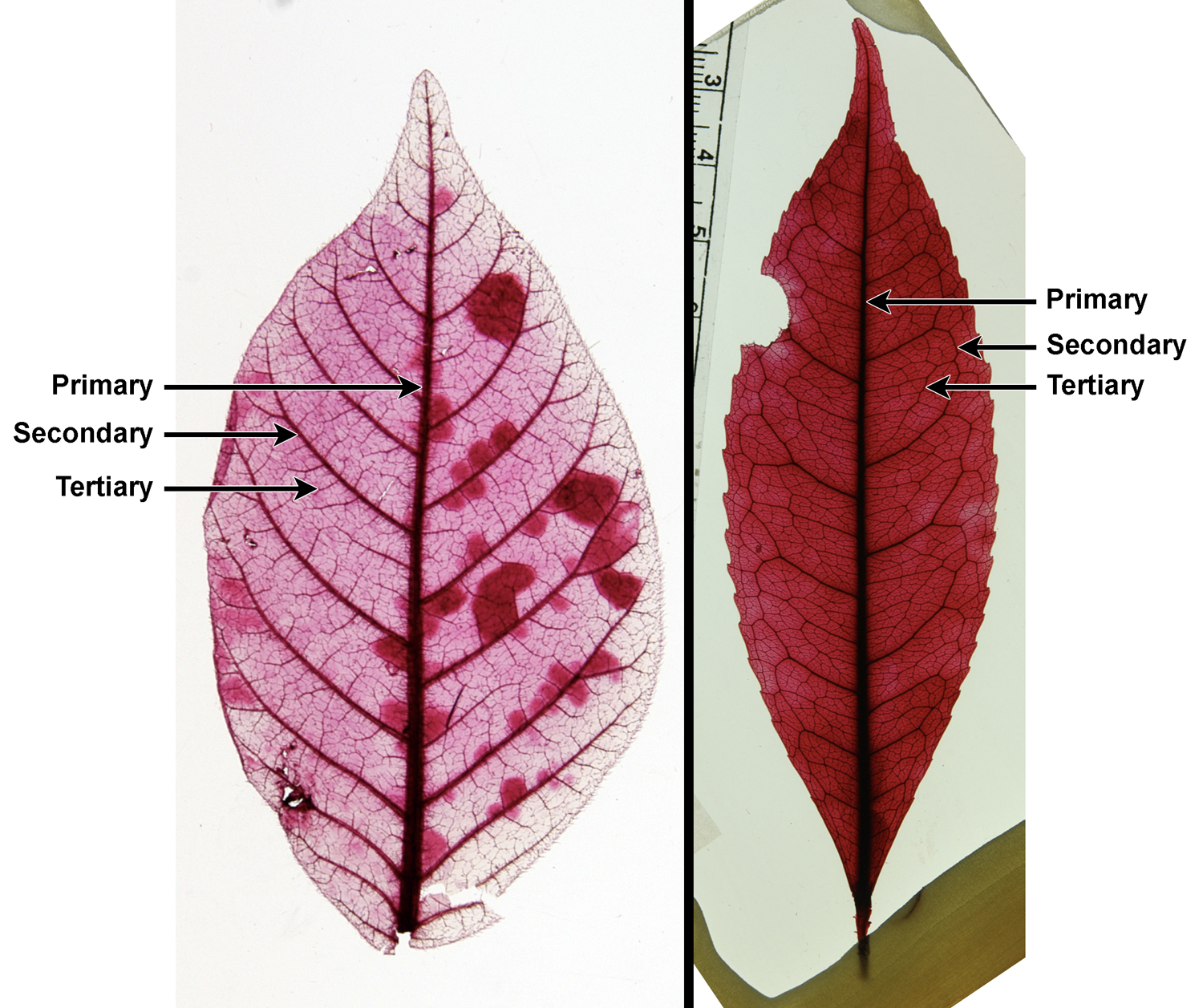
Describing leaf venation patterns is one of the most important and potentially challenging parts of describing many dicot leaves. Veins are sorted into categories by thickness. The thickest veins are the primary veins, the next thickest the secondary veins, the next thickest the tertiary veins, and so on. The number of vein orders a leaf has as well as the organization of each order of veins is important to describing and potentially identifying an angiosperm leaf. The brief overview below mostly follows the Manual of Leaf Architecture, although in simplified form.
Primary venation
Primary veins are the thickest and most prominent vein or veins in the leaf. Secondary veins are the next-thickest veins. The primary veins or the primary and secondary veins together determine the major venation pattern of the leaf, which can broadly be divided into pinnate or palmate.
Pinnate venation
If one primary vein is present and it divides the leaf blade from base to apex, then the primary vein is a midvein. If secondary veins (major veins that are slightly thinner than the midvein) depart from either side of the midvein, then the overall major venation pattern is called pinnate (remember, "pinnate" refers to a feather-like organization). Thus, the term pinnate encompasses the pattern of both primary and secondary veins together.

Palmate venation
If at least three primary veins emerge from the base of the leaf or slightly above the leaf base, the primary venation pattern is palmate (remember, palmate is organized like the fingers radiating from the palm of a hand). Note that some sources distinguish between palmate venation and ternate or trinerved venation; in that case, leaves with ternate or trinerved venation have three primary veins radiating at or just above the leaf base, whereas leaves with palmate venation have at least four primary veins.
Palmate venation is divided into a number of different categories depending on the course of the primary veins. Note that the terms for categories of palmate venation end with "-dromous"; this ending derives from the Greek word for course or running. Thus, these terms describe the course of the veins. Some examples of palmate venation include:
- Actinodromous (Greek, aktis = ray): The primary veins radiate from one point and proceed on a relatively straight course toward the leaf margin.
- Palinactinodromous (Greek, palin + aktis = again ray): Like actinodromous, except the lateral primary veins branch above the leaf base.
- Acrodromous (Greek, ákros = end or peak): The major veins arise from one point; the lateral primary veins and/or thick secondary veins curve outward and then come together near the leaf tip.
- Campylodromous (Greek, kampylos = bent or curved): The lateral primary veins are recurved (the veins curve toward the leaf base then turn upward), coming together near the leaf tip. Note that the lateral primary veins may branch above the leaf base.


Secondary venation
Secondary veins are veins that are narrower than the primary veins, but thicker than the tertiary veins that fill in the lamina (blade) of the leaf (see below). The most prominent secondary veins are often those branching off the sides of the primary vein(s); they are called major or costal (Latin costa = rib) secondary veins. These secondary veins are categorized based on their course (straight or curved) and how they end (for example, looping to join other secondary veins, ending at the leaf margin, or thinning out as they approach the leaf margin).
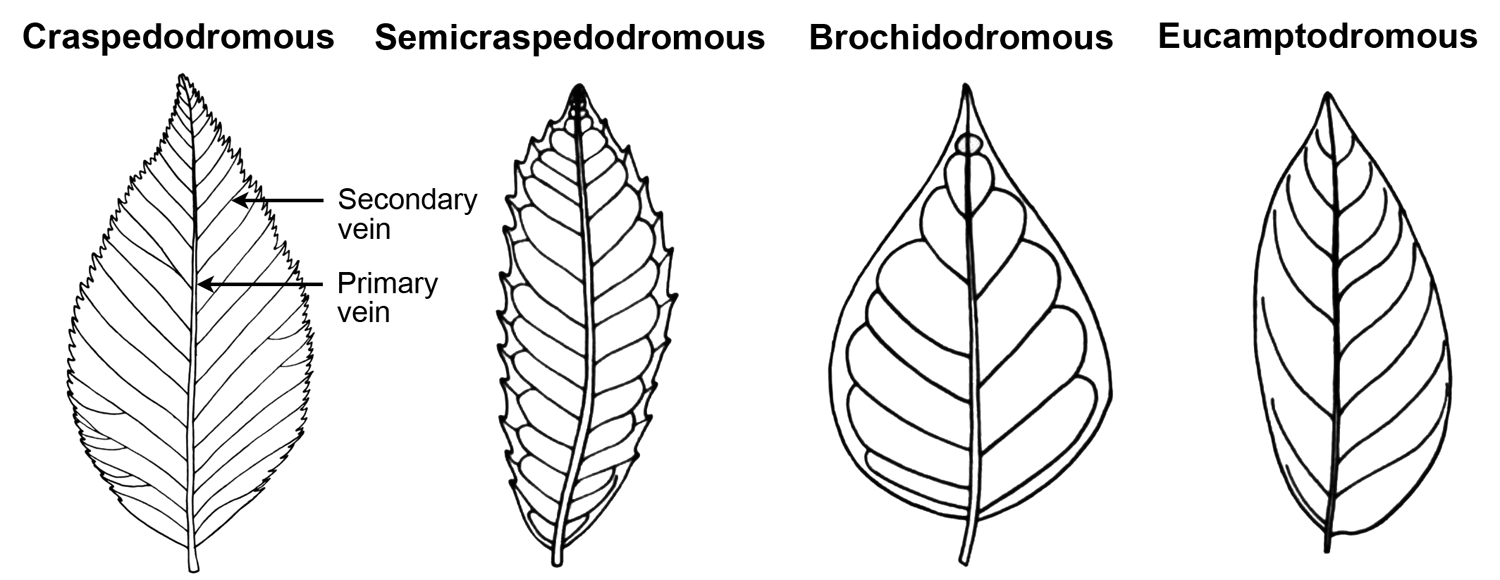
The terms for categories of major secondary venation end with "-dromous"; as noted above, this ending derives from the Greek word for course or running. Thus, these terms describe the course of the major secondary veins. Examples of major secondary venation types include:
- Craspedodromous (Greek, kraspedon = edge or border): Secondary veins are relatively straight and end at the leaf margin without looping.
- Semicraspedodromous: Each secondary vein divides. One half of the division curves upward and connects with the secondary vein above it. The other half of the division travels to the leaf margin, often ending in tooth.
- Brochidodromous (Greek, brochos = loop): Each secondary vein curves and fuses with the secondary vein immediately above it.
- Eucamptodromous (Greek, eu + kamptos = true curved): Secondary veins curve upward but do not connect with one another to form loops. The secondary veins diminish in thickness as they near the leaf margin.

Tertiary venation
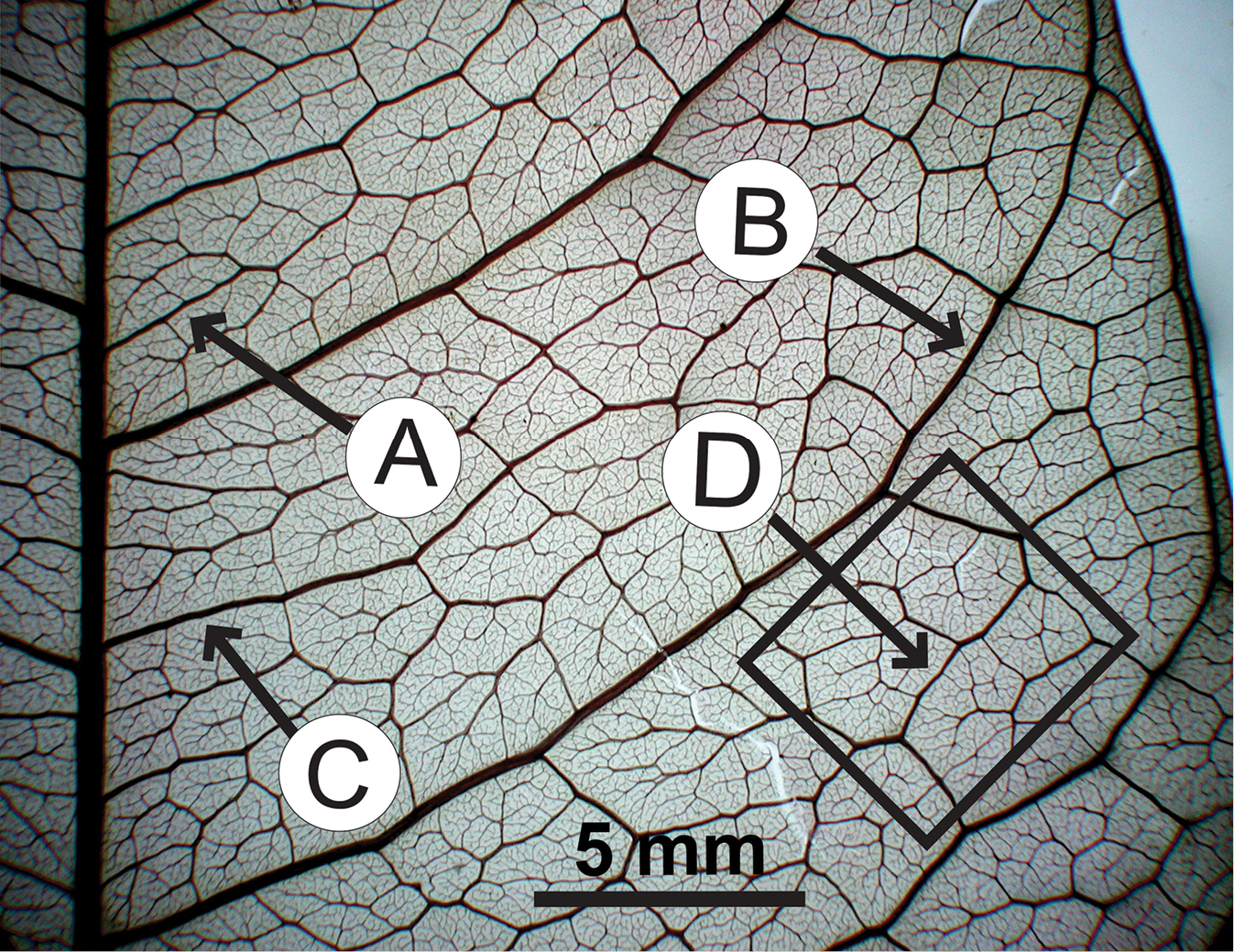
Tertiary veins are thinner than secondary veins, and they are part of the network of veins that fill in the spaces between major veins in the leaf lamina (blade). Tertiary veins that occur between the major secondary veins are known as intercostal (Latin inter + costa = between rib) veins. Three major categories of intercostal tertiary venation are recognized:
- Percurrent: Tertiary veins run between secondary veins without branching or fusing with one another. Percurrent tertiary veins are not necessarily straight and may be curved or have angles in them.
- Ramified: Tertiary veins branch but do not anastomose (fuse or join).
- Reticulate: Tertiary veins fuse with one another to form a network (reticulum) between the major veins.

Higher-order veins & areoles
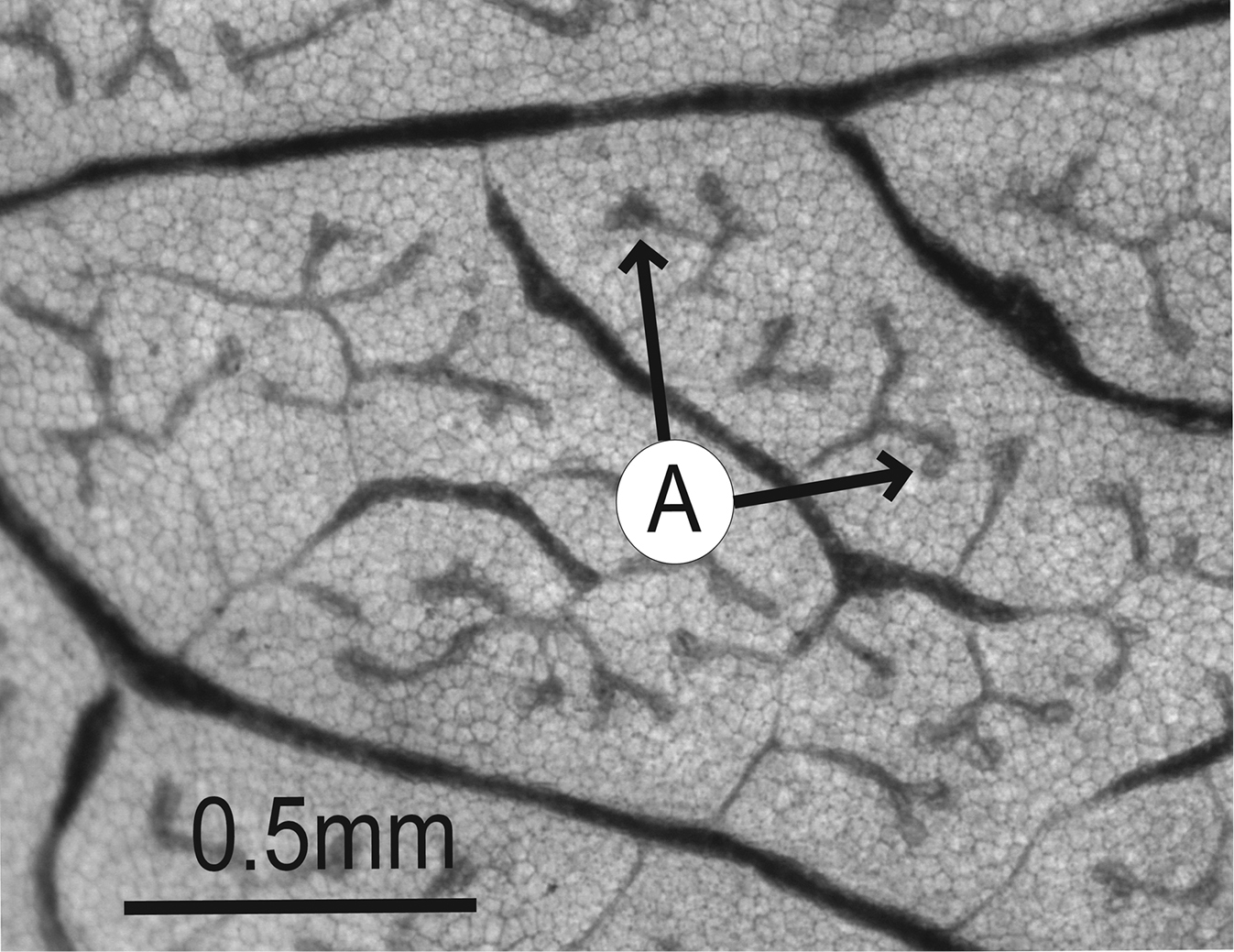
Leaves may have vein orders above tertiary venation, including quaternary veins (fourth-order), quinternary veins (fifth-order), and even higher orders. Like tertiary veins, these veins fill in the spaces between the major veins of the leaf. In many angiosperm leaves, the higher-order veins anastomose (fuse) and form enclosed regions known as areoles. The areoles may or may not have branching veins within them. Branching veins with an areole that do not fuse to any other veins are known as freely ending veinlets (FEVs).


Parallel venation patterns
Monocots are angiosperms with embryos that have one cotyledon (seed leaf). Examples of monocots are grasses, palms, bananas, and gingers. Monocot leaves often have parallel or modified parallel venation. In a parallel-veined leaves, the major veins run parallel to one another and converge at the tip of the leaf. The major parallel veins are often connected by finer, less conspicuous veins that run between them. Monocot leaves often do not have a distinct stalk (petiole) and are often linear or strap-like in form. Leaves of plants like grasses (family Poaceae), sedges (family Cyperaceae), and daylilies (Hemerocallis fulva) fit the typical monocot pattern.
Two types of parallel or modified-parallel monocot leaf venation are included in the Manual of Leaf Architecture: parallelodromous and campylodromous. In parallelodromous venation, at least two (but often many) primary veins emerge from the base of the leaf, coming together near the leaf tip. In campylodromous venation, venation is palmate and the lateral primary veins are recurved (curve toward the leaf base before turning upward), coming together at the leaf tip. Note that the definition of campylodromous—specifically, whether the primary veins emerge from one point or next to one another from the leaf base—differs slightly among sources; leaves fitting both definitions are shown on this page (see the Dioscorea amazonum leaf above and the Maianthemum dilatatum leaf below).
Some monocot leaves have other modifications of the parallel pattern. In fan-shaped leaves like those of the palmetto (Sabal, a type of palm), the major veins radiate either from the base of the leaf or from a short midrib. Thus, the venation may be described as palmate and parallel. In monocots like bananas (Musa) and gingers (Zingiber), each leaf has a large midvein with many lateral veins to either side. The lateral veins are parallel to one another. This type of venation pattern is called penni-parallel.




Leaf margins
The leaf margin is the edge of the leaf. Leaf margins can come in a variety of types. An entire margin is smooth and uninterrupted.
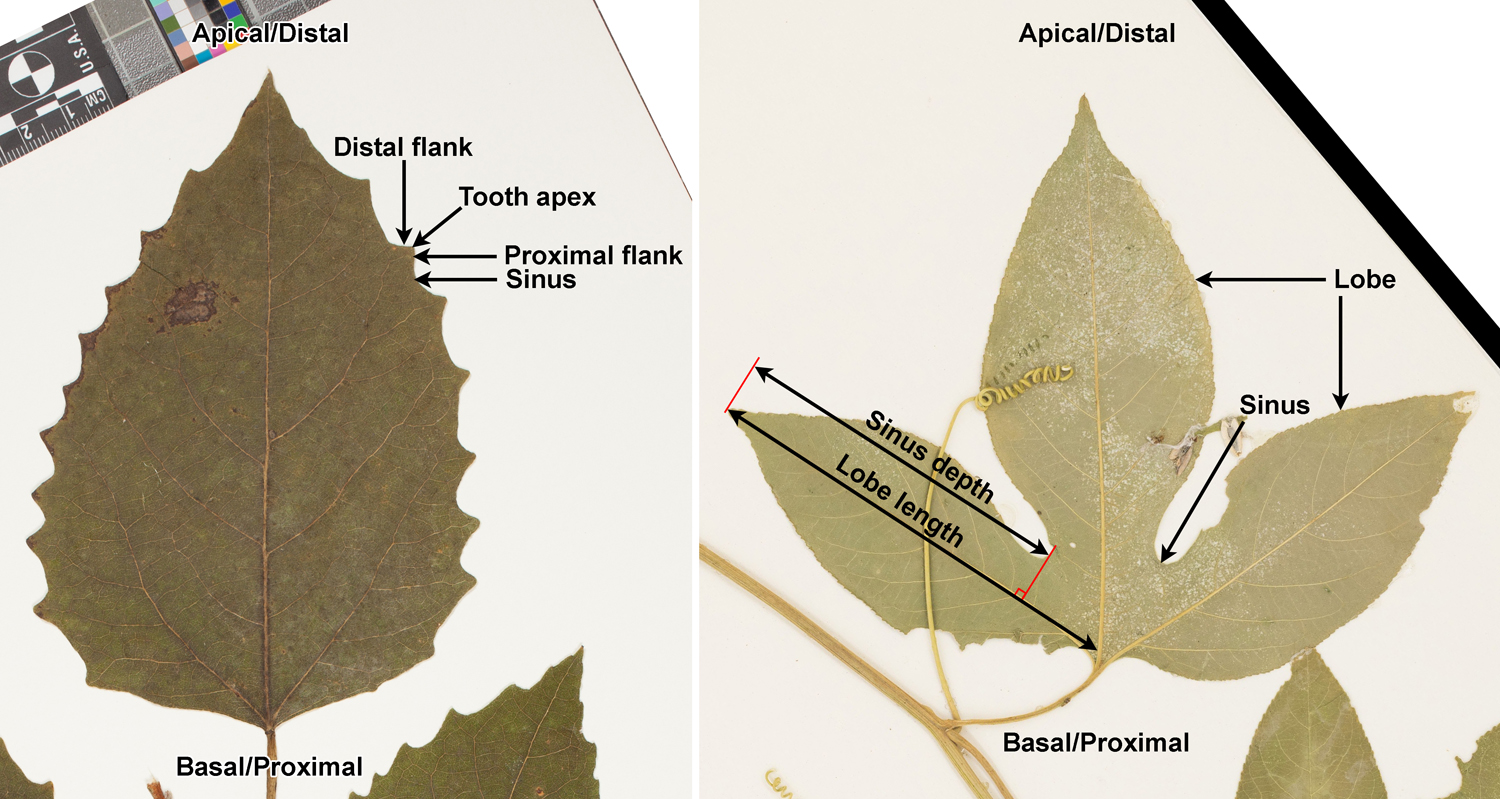
Margins that are not entire may have teeth, lobes, or both teeth and lobes. A toothed margin has teeth—essentially, it has an edge that resembles the blade of a saw. Teeth may be large or small, pointed or rounded, and have different shapes. The upper side of the tooth—meaning the side of the tooth facing the apex of the leaf—is know as the distal flank. The lower side—meaning the side of the tooth facing the base of the leaf—is known as the proximal flank. The valleys between teeth are called sinuses.
Lobes are larger than teeth and are divided by deeper sinuses. Usually, the difference between teeth and lobes is clear. However, large teeth and shallow lobes can be difficult to distinguish. The 2009 edition of the Manual of Leaf Architecture defines a lobe based on the depth of the adjoining sinus on the upper (apical or distal) side of the lobe. The depth of the sinus must be at least 25% the total length of the projection for a projection to be classified as a lobe. (The length of the projection is measured from the tip of projection to the midvein of the leaf.) Other sources, however, may use a different cut-off.

Leaf teeth come in a variety of shapes. In modern plants, these are usually defined using a series of terms that describe a combination of tooth size and tooth tip shape (for example, rounded or pointed). Three basic types are serrate (teeth sharp, tooth apex pointed apically), dentate (teeth sharp, tooth apex pointed outward), or crenate (teeth with rounded apices) to describe the leaf margin.
Additionally, teeth may be compound. Compound teeth are teeth that have more than one projection (point), a large projection and one or more smaller projections that are usually on its distal flank. On a leaf with compound teeth, the primary teeth are the largest teeth, which are typically fed by the most prominent veins. Smaller teeth—sometimes called secondary teeth—are the smaller points on the flanks of the primary teeth.

In paleobotanical studies that use the physiognomy (essentially, architecture and/or dimensions) of woody dicot leaves to estimate paleoclimate, leaf margin type may be one of the leaf characters scored. In these studies, leaves are divided into untoothed and toothed. The categories are as follows:
- Untoothed leaves: Leaves with entire margins and leaves with lobed margins and no teeth.
- Toothed leaves: Leaves with teeth, whether unlobed or lobed.
The reason for this division is because leaf teeth are an important climate indicator. Woody dicots with untoothed leaf margins make up a higher percentage of species in warmer-climate floras and a lower percentage in cooler-climate floras.

Selected references & further reading
Note: Free full text is made available by the publisher for items marked with a green asterisk.
Academic articles & book chapters
* Allen, S.E., A.J. Lowe, D.J. Peppe, and H.W. Meyer. 2020. Paleoclimate and paleoecology of the uppermost Eocene Florissant flora (Central Colorado, USA), ver. 2. PaleorXiv. [Preprint] https://doi.org/10.31233/osf.io/xpm26
Allen, S.E., A.J. Lowe, D.J. Peppe, and H.W. Meyer. 2020. Paleoclimate and paleoecology of the latest Eocene Florissant flora (Central Colorado, USA). Palaeogeography, Palaeoclimatology, Palaeoecology. https://doi.org/10.1016/j.palaeo.2020.109678
* Angiosperm Phylogeny Group [APG] IV. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. https://doi.org/10.1111/boj.12385
* García-Gutiérrez, E., F. Ortega-Escalona, and G. Angeles. 2020. A novel, rapid technique for clearing leaf tissues. Applications in Plant Sciences 8: e11391. https://doi.org/10.1002/aps3.11391
Hickey, L.J. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17–33. https://doi.org/10.1002/j.1537-2197.1973.tb10192.x
* Hickey, L.J., and J.A. Wolfe. 1975. The bases of angiosperm phylogeny: Vegetative morphology. Annals of the Missouri Botanical Garden 62: 538–589. https://doi.org/10.2307/2395267
* Jud, N.A., M.A. Gandolfo, A. Iglesias, and P. Wilf. 2017. Flowering after disaster: Early Danian buckthorn (Rhamnaceae) flowers and leaves from Patagonia. PLoS ONE 12(5): e0176164. https://doi.org/10.1371/journal.pone.0176164
* Kvaček, Z., V. Teodoridis, and T. Denk. 2019. The Pliocene flora of Frankfurt am Main, Germany: taxonomy, palaeoenvironments and biogeographic affinities. Palaediversity and Palaeoenvironments. https://doi.org/10.1007/s12549-019-00391-6
* Mitchell, J.D., and D.C. Daly. 2015. A revision of Spondias L. (Anacardiaceae) in the Neotropics. PhytoKeys 55: 1–92. https://doi.org/10.3897/phytokeys.55.8489
* Royer, D.L., P. Wilf, D.A. Janesko, E.A. Kowalski, and D.L. Dilcher. 2005. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany 92: 1141–1151. https://doi.org/10.3732/ajb.92.7.1141
* Vasco, A., M. Thadeo, M. Conover, and D.C. Daly. 2014. Preparation of samples for leaf architecture studies, a method for mounting cleared leaves. Applications in Plant Sciences 2: 1400038. https://doi.org/10.3732/apps.1400038
Wilf, P., K.C. Nixon, M.A. Gandolfo, and N.R. Cúneo. 2019. Eocene Fagaceae from Patagonia and Gondwanan legacy in Asian rainforests. Science 364: eaaw5139. https://doi.org/10.1126/science.aaw5139
Books, textbooks & manuals
* Ellis, B., D.C. Daley, L.J. Hickey, K.R. Johnson, J.D. Mitchell, P. Wilf, and S.L. Wing. 2009. Manual of Leaf Architecture. Comstock Publishing Associates, Cornell University Press, Ithaca, New York. Free PDF on ResearchGate (shared with Creative Commons Attribution-NonCommercial license, CC BY-NC 4.0): https://www.researchgate.net/publication/270216727_Manual_of_Leaf_Architecture
Evert, R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York.
Harris, J.G., and M.W. Harris. 2001. Plant Identification Terminology, an Illustrated Glossary, 2nd ed. Spring Lake Publishing, Spring Lake, Utah.
* Leaf Architecture Working Group (A. Ash, B. Ellis, L.J. Hickey, K. Johnson, P. Wilf, and S. Wing). 1999. Manual of Leaf Architecture: Morphological description and categorization of dicotyledonous and net-veined monocotyledonous angiosperms. Smithsonian Institution, Washington, DC. Free PDF from P. Wilf, Pennsylvania State University: https://personal.ems.psu.edu/~pdw3/1999_MLA.pdf
Simpson, M.G. 2010. Plant Systematics, 2nd ed. Academic Press, Burlington, Massachusetts.
Websites
* CLAMP leaf character state definitions and scoring. CLAMP online. http://clamp.ibcas.ac.cn/CLAMP_Definitions.html
* Cleared Leaf Image Database (J. Weitz, A. Bucksch, A. Das, C. Price): http://www.clearedleavesdb.org/
* Fisher, J. 2007. Leaf or branch. Fairchild Tropical Botanic Garden Virtual Herbarium: http://www.virtualherbarium.org/gardenviews/LeafOrBranch.html
* The National Cleared Leaf Collection, Yale Peabody Museum and Smithsonian National Museum of Natural History (L.J. Hickey and S. Hu). https://collections.peabody.yale.edu/pb/nclc/
* Trees of Wisconsin. Herbarium, Cofrin Center for Biodiversity, University of Wisconsin-Green Bay. Page with images of simple and compound leaves, showing differences. Link
* Wilf, P., K.C. Nixon, M.A. Gandolfo, and N.R. Cúneo. 2019. Image archive for: Eocene Fagaceae from Patagonia and Gondwanan legacy in Asian rainforests. Figshare. https://doi.org/10.6084/m9.figshare.7480007.v1
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
Page first online: 24 August 2021; last updated: 28 September 2021.



