Chapter by:
Warren D. Allmon* and Jonathan R. Hendricks* (Paleontological Research Institution, Ithaca, New York). Both authors contributed equally to this work and authorship is alphabetical.
This is a heavily-revised second edition of this chapter and was last updated by the authors on September 16, 2021.
Image above: A variety of modern and fossil gastropod shells that illustrate some of the many shapes of marine and nonmarine (land and freshwater) snails. Although these species are relatively large, most gastropods on Earth are quite tiny, less than the size of a mini chocolate chip. Note that abalone in the top middle is a marine snail, even though it looks like half a bivalve.
Chapter citation:
Allmon, W. D., and J. R. Hendricks. 2021. Gastropoda (Revised). In: The Digital Encyclopedia of Ancient Life. https://www.digitalatlasofancientlife.org/learn/mollusca/gastropoda/.
Acknowledgments:
We are grateful to Brendan Anderson, Paula Mikkelsen, Alex Nützel, and Christi Sobel for assistance with figures, to Dana Friend and Sojeet Sharma for help with extinction data, Vicky Wang for assistance with locating specimens, and Paula Mikkelsen, Alex Nützel, and Rebecca Rundell for comments on the text.
Chapter contents:
Class Gastropoda: Introduction, Morphology, Anatomy, and Life History ←
– 1. Phylogeny and Classification of Extant Gastropoda
– 2. Fossil Record of Gastropoda
– 3. References and Further Reading
– 4. Silly Snail Stuff
Virtual Collection:
A virtual collection of interactive 3D models of gastropod specimens is associated with this chapter.
Snapshot: Gastropoda
Taxonomy: Phylum Mollusca, Class Gastropoda
Common names of representatives: Snails, slugs, conchs, whelks, periwinkles, abalone, limpets, etc.
Habitats: Marine (salt water), freshwater (lakes and streams), and terrestrial (on land).
Feeding types: Herbivorous (algae or plant eaters), carnivorous predators (meat eaters/hunters), detritivores (feed on dead organic matter), scavengers (feed on dead animals), and parasitic (feed on living animals).
Geological range: Cambrian Period (541 million years ago) to Recent (today).
Clade defining features: Torsion during development of larvae, head present, shell (if present) univalved (single shell), operculum (if present) located on the foot.
Overview
Gastropods are the second largest class of animals (after the Insecta)—with 40,000–90,000 living species and at least 13,000 extant and fossil genera (Ponder and Lindberg, 2020)—and are also one of the most evolutionarily successful groups in the variety of ecosystems and habitats that they occupy. They live in the world’s oceans, freshwater lakes and streams, and terrestrial ecosystems, including deserts, mountains, backyards, and beaches. Their shells range in size from almost a meter to less than a millimeter. Some are algae-eating herbivores, some eat detritus (detritivory), some are parasites, and others are carnivorous predators, including of fish. Their reproductive strategies are similarly diverse; some species have separate sexes, some are hermaphrodites, and others like Crepidula switch sex during their lives. While gastropods have many different shell shapes (or no shells at all) and lifestyles, they are united by undergoing a process called torsion during their development (see below). In some snails, fertilization occurs internally through copulation, but in others it involves external spawning. Most gastropods have a single shell, but many have no shell at all. Their shells have left behind an abundant Cambrian to Recent (i.e., today) fossil record that has been the focus of many paleobiological studies.
The name “Gastropoda” comes from the Greek roots “gastro” (= stomach) and “pod” (= foot). Snails were given this name because many have the appearance of crawling around on their stomachs.

The foot of a cowrie (Cypraeidae) is visible as it crawls on a pane of aquarium glass. Photograph by Jonathan R. Hendricks.
Gastropod Morphology, Anatomy, and Life History
The shells of snails are lovely natural objects and are commonly featured in artwork and beach-themed decorating motifs. They are frequently found at the shore and collectors have coveted them for centuries. People tend to be less familiar, however, with the animals that build and reside within these shells for their entire lives. Because well-preserved shells record almost the entire life of an individual animal, they are very useful for investigating the relationship between evolution and development, as well as paleoecology. Most descriptions of extant (or, living) shelled gastropods, particularly those made decades or centuries ago, are based only upon shell morphology. Thus, the shells of gastropods are also very important for taxonomy and classification. Yet, it is the soft-animal inside that is responsible for building the shells that people treasure. Understanding the animal and how it lives helps us to make sense of its shell.

Shells of the cowrie Naria miliaris. Compare with image below of the living animal. Specimens from Sarawak, Borneo, collected at 80 m depth (PRI 85880). Photograph by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
Soft-Part Anatomy
External Anatomy
Most gastropods have bodies that consist of several basic parts. Snails crawl upon a fleshy foot (see image above) that spans the length of their body; movement occurs via muscle action and hydrostatic pressure. The soft-tissue mantle covers the internal organs and is used to build the shell (when present). All gastropods have a head, which has a mouth, sensory structures such as tentacles or siphons, and eyes, which are sometimes at the ends of stalks.

Two individuals of the miliaris cowrie (Naria miliaris); compare with the image above that shows only the shell of this species. In cowries, the mantle commonly extends over the shell, giving it a glossy appearance; in this species, the mantle is covered by fleshy protrusions. Note the tube-shaped siphon and tentacles at the front of each animal (top of photo). Photograph by Ria Tan (Flickr; Creative Commons Attribution-NonCommercial-NoDerivs 2.0 Generic license).
The eyes of some gastropods are simple and can only distinguish light from dark, while others—such as those of conchs like Lambis—are complex and have lenses that are capable of forming images (Seyer, 1994).

Digestive System
Food enters the gastropod body through the mouth, which may be positioned at the end of an extended structure called the rostrum or proboscis in carnivorous marine gastropods. All gastropods have a specialized structure called a radula that is used to obtain food. The radula is a ribbon-like band that is covered by rows of bristle-shaped teeth that are made of chitin.
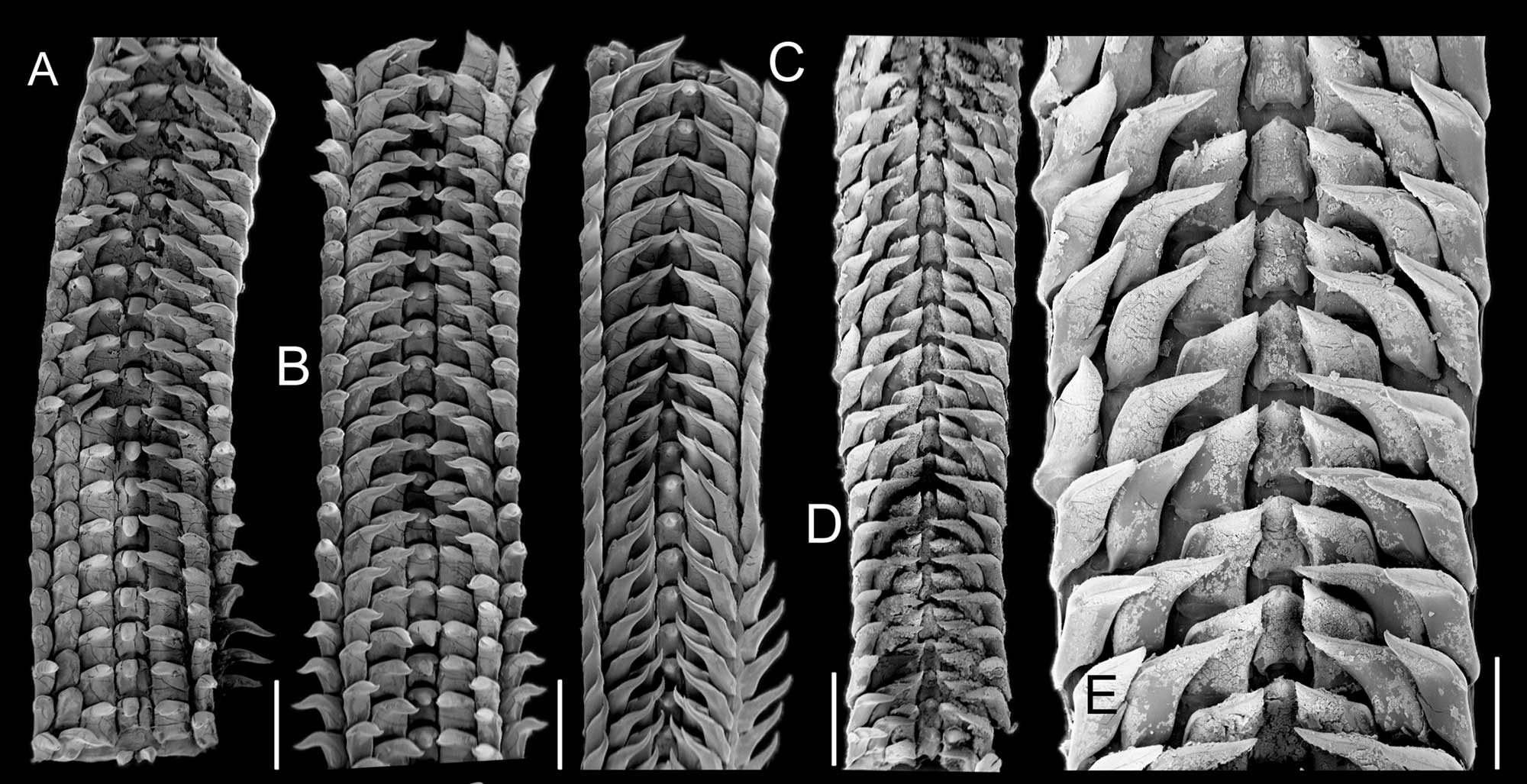
Examples of cowrie radulae (Macrocypraea spp.). Scanning electronic microscope images are from Simone and Cavallari (2020, fig. 4 in PLoS ONE; Creative Commons Attribution 4.0 International license; image modified from original). Original caption: "(A) M. mammoth paratype MZSP 137546; (B-C) M. mammoth holotype. (D) M. cervus ANSP 93276. (E) Same, detail. scales = 32–35: 1 mm, 36: 0.5 mm."

Scanning electron microscope image of the radula of the heterobranch bubble snail Bulla occidentalis from the Indian River Lagoon, Florida. Image by Paula M. Mikkelsen for the Digital Atlas of Ancient Life project.
The radula moves forward and backward to scrape food, such as algae, from the surfaces of rocks or vegetation.

Gastropod radular anatomy. Labels: e, esophagus; m, mouth; mx, mandible; o, odontophore; op, odontophore protractor muscle; r, radula; rp, radula protractor muscle; rr, radula retractor muscle. Image by "Debivort" (Wikimedia Commons; Creative Commons Attribution-Share Alike 30 Unported license; image cropped and orientation modified from original).
"Trochus snail feeding" by Digital Atlas of Ancient Life (YouTube).
In some gastropods, such as cone snails, the radula has been modified by evolution into a harpoon-shaped syringe that is used to capture and immobilize prey using complex neurotoxins.
"Watch These Cunning Snails Stab and Swallow Fish Whole" by Deep Look (YouTube).
Food passes from the mouth to the stomach (which processes food and sometimes features a chitinous lining or grinding plates) and then on to the digestive gland, which converts food to nutrients. Waste is shaped into fecal pellets by the intestine and these pellets then exit the gastropod body through the anus.
Respiration
Respiration in marine gastropods is usually achieved with one or two gills, called ctenidia, that are located inside of the mantle cavity, a space formed by a fold in the mantle. There is considerable variation among clades in the arrangement of gills. For example, some taxa have lost their primary ctenidia, which have been replaced by secondary gills. Many nudibranchs and microscopic snails (those that are very small as adults) lack gills entirely and absorb oxygen directly through the integument (outer surface of the body, analogous to our skin). Respiration in terrestrial gastropods is achieved using a portion of the mantle cavity that is highly vascularized, essentially serving as a lung.
Shell Coiling and Orientation
The image below shows a fossil specimen of Strombus in life position.
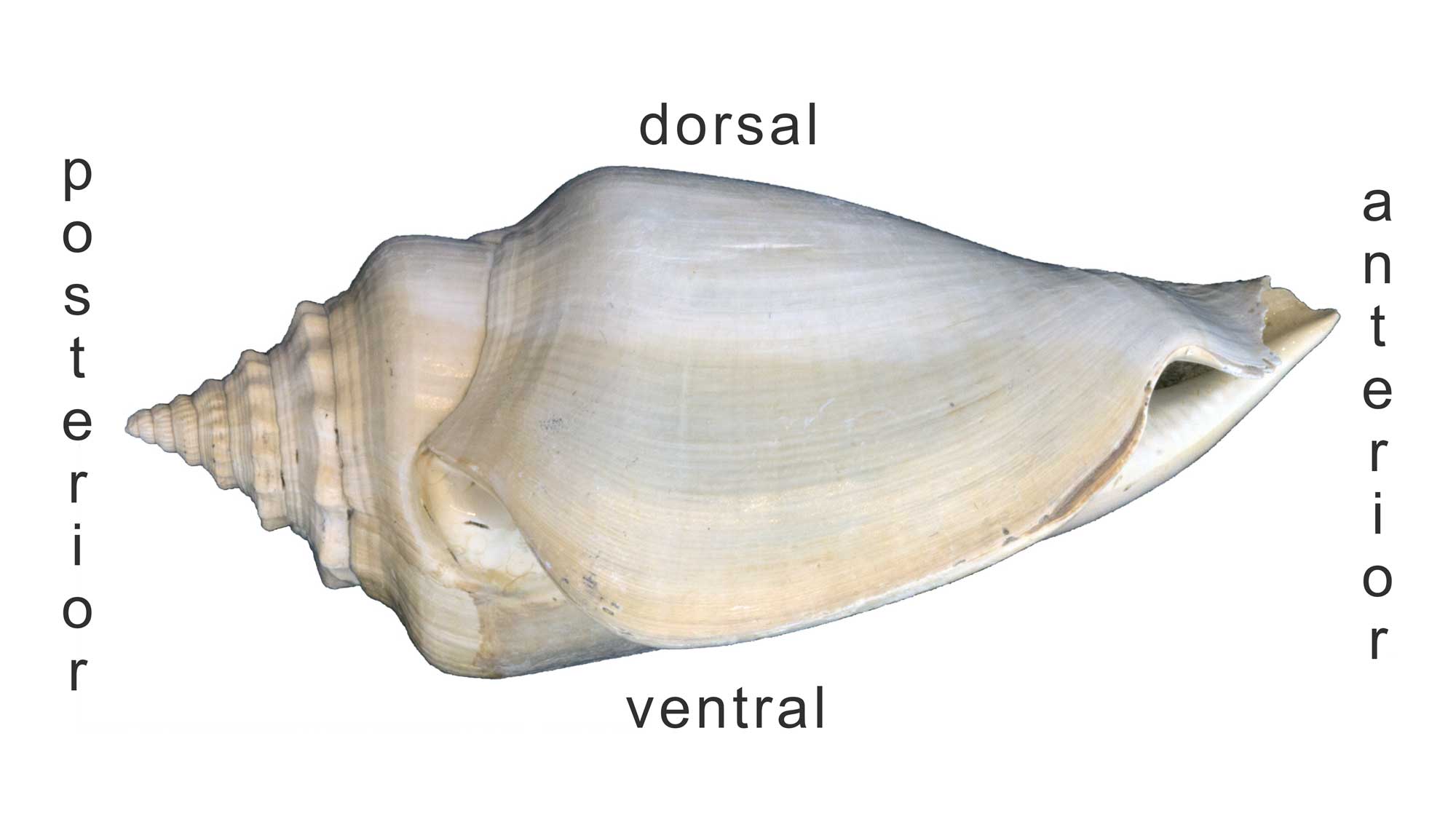
Side-view of a Plio-Pleistocene Strombus shell with the posterior, anterior, dorsal, and ventral sides identified. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
The opening of the shell (aperture) defines the ventral surface; the opposite side (or, top) is the dorsal surface. Because gastropod shells grow by coiling about an axis, parts of the shell that were once “ventral” eventually become oriented dorsally as new shell material is deposited at the edge of older material. The anterior side of the shell is the “front end” (i.e., is located near the head, so the snail crawls in that direction); the posterior side of the shell usually bears the spire (if present).
When describing gastropod shells, it is often useful to characterize the positions of certain features relative to the apex of the shell or its axis of coiling (see above). The Latin prefex “ab-” means “away from.” Thus, features that are positioned away from the apex are called abapical and features that are positioned away from the axis of coiling are called abaxial. The Latin prefex “ad-” means just the opposite: “toward.” Therefore, a structure positioned toward the apex is adapical and a structure positioned toward the axis of coiling is adaxial.
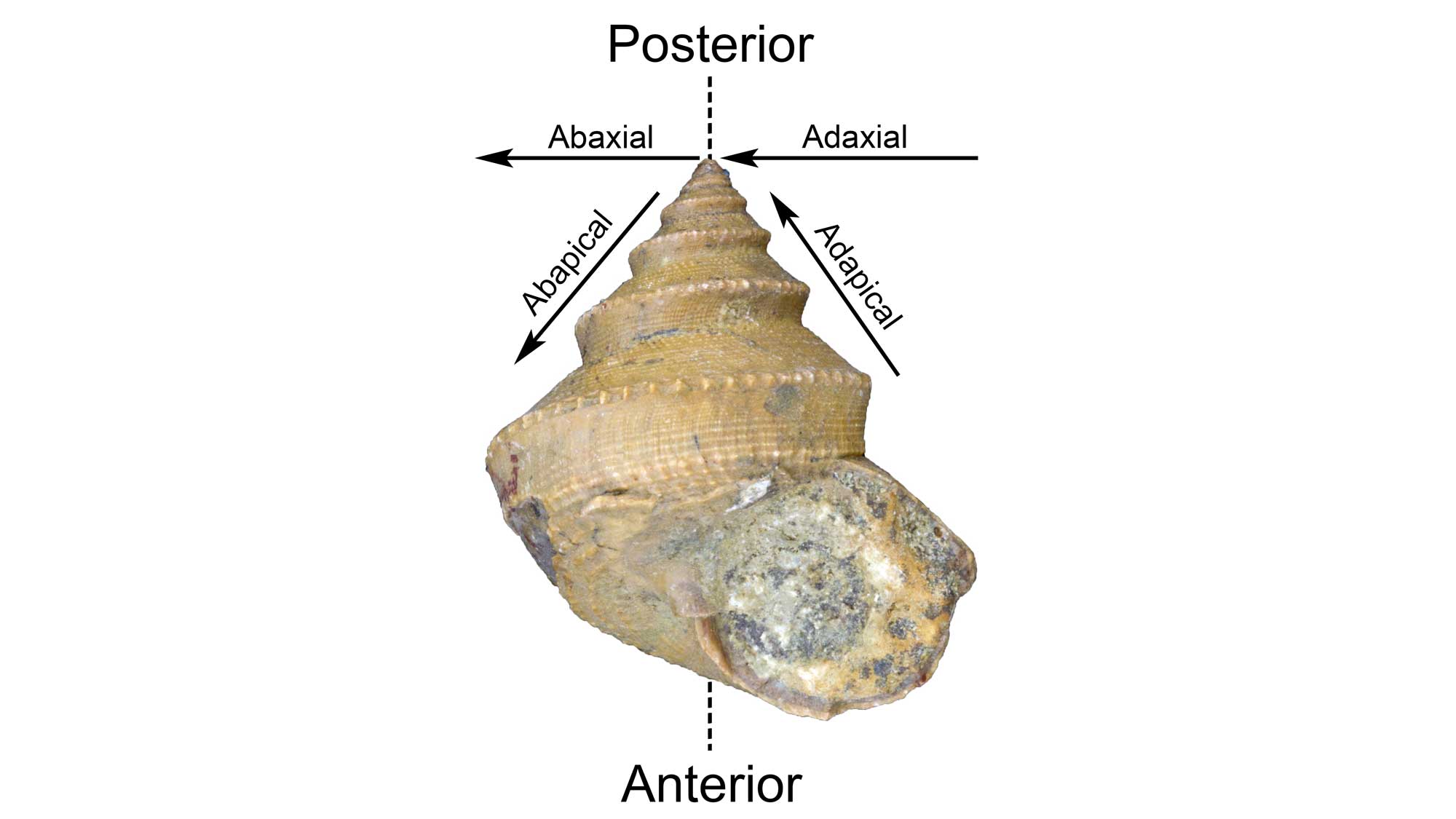
Positional terminology for a gastropod shells. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life Project. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York.
Although some gastropod shells have non-coiled limpet-shapes, most are coiled. Cinnamon-bun shaped planispiral gastropod shells coil in a single plane (two dimensions) and are relatively rare among modern species.
Much more common are conispiral (helical) shells that coil along the coiling axis in three dimensions. Conispiral shells exhibit chirality, or a directionality to the way the aperture coils around the axis. Most gastropod species that have ever lived have shells that are dextral: when positioned with their apex pointing upward, their apertures open to the right. Far fewer species exhibit sinistral, or left-handed shell coiling (aperture opening on the left side).

"Fossils@Home #3: Left-Handed Snails" by Paleontological Research Institution (YouTube).
Shell chirality is determined by a single gene in gastropods and is evident at a cellular scale very early in development.
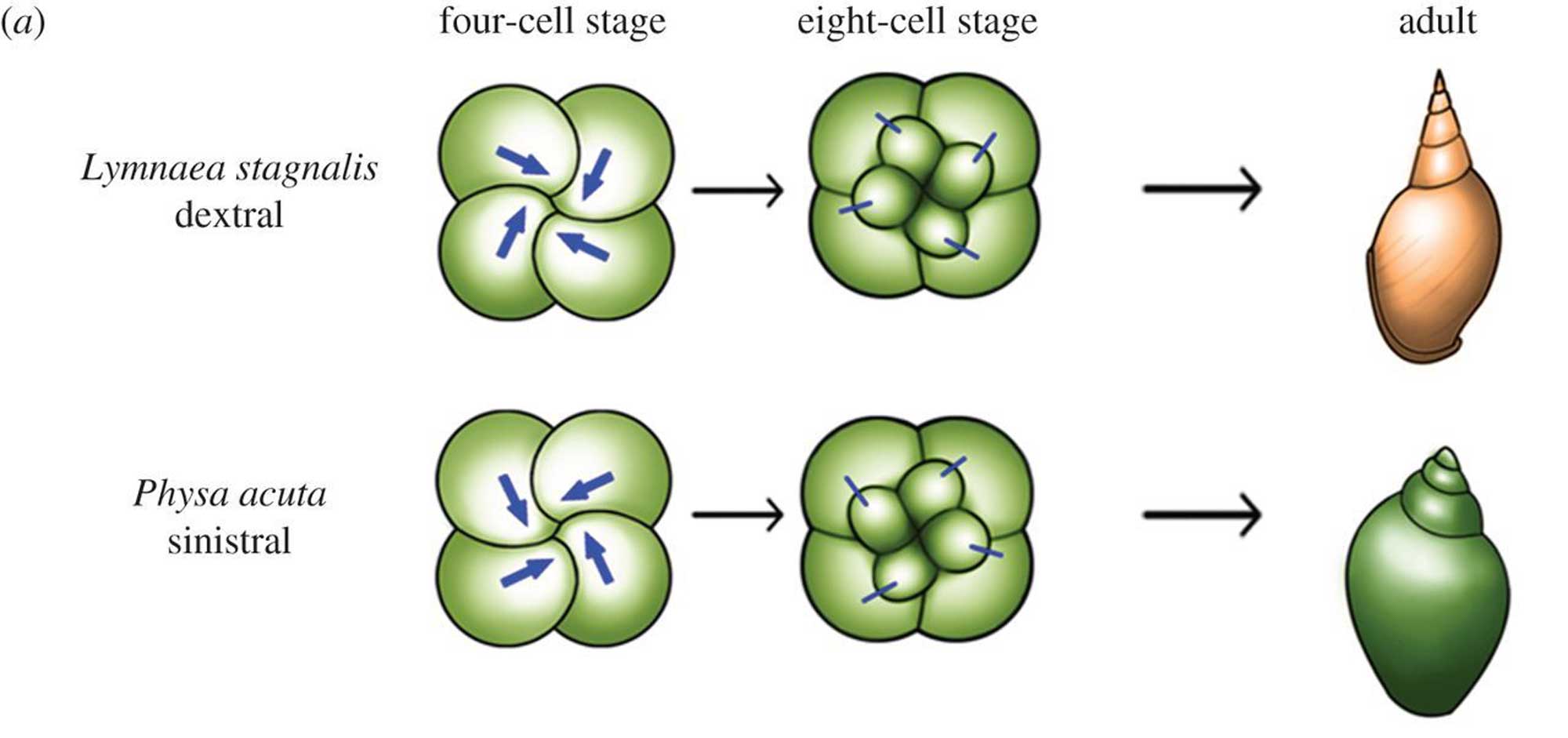
Differences between dextral and sinistral snails become evident very early on in development. Image from fig. 5 in Inaki et al. (2016, Philosophical Transactions of the Royal Society B; Creative Commons Attribution 4.0 International license; image cropped and resized relative to original). Original caption: "Figure 5. Cell chirality in snails and C. elegans. (a) Upper: in Lymnaea, the blastomere spindles slant clockwise as viewed from the animal pole at the four-cell stage, and blastomeres are rearranged clockwise at the eight-cell stage. Bottom: Pulmonata is a snail species with counter clockwise-coiling shells and internal organs that mirror those of Lymnaea. In Pulmonata, blastomere spindles slant anticlockwise as viewed from the animal pole, resulting in a counter clockwise blastomere rearrangement at the eight-cell stage. In both cases, blastomere chirality determines the shell coiling direction and LR asymmetry of the body."
In rare instances, the coiling direction of a snail may be different from that of its parents. This amounts to essentially instantaneous speciation because snails of opposite coil generally cannot orient their bodies for reproduction. One such oppositely-coiling gastropod, a sinistral garden snail named Jeremy, reached global fame after a worldwide search was undertaken to find him a similarly-coiled mate.
"This Backward Snail Just Wants to Find Love" by Planet Earth (YouTube).
The bodies of gastropods are asymmetrical, just like your own (consider the asymmetry of your own internal organs, for example the shape and position of your heart and liver). Gastropods may or may not have a body chirality that matches their shell, and this leads to a complex variety of conditions. When the chirality of the shell matches the chirality of a snail’s body, the descriptor orthostrophy is used (e.g., dextral orthostrophy, the most common state in gastropods). When the chirality of the shell does not match the chirality of a snail’s body—a very rare condition—the descriptor hyperstrophy is used. In fossil gastropods, the coiling direction of the soft body can only be inferred from the coiling direction of the operculum (if there is one), or, in some cases from the shape of the aperture.
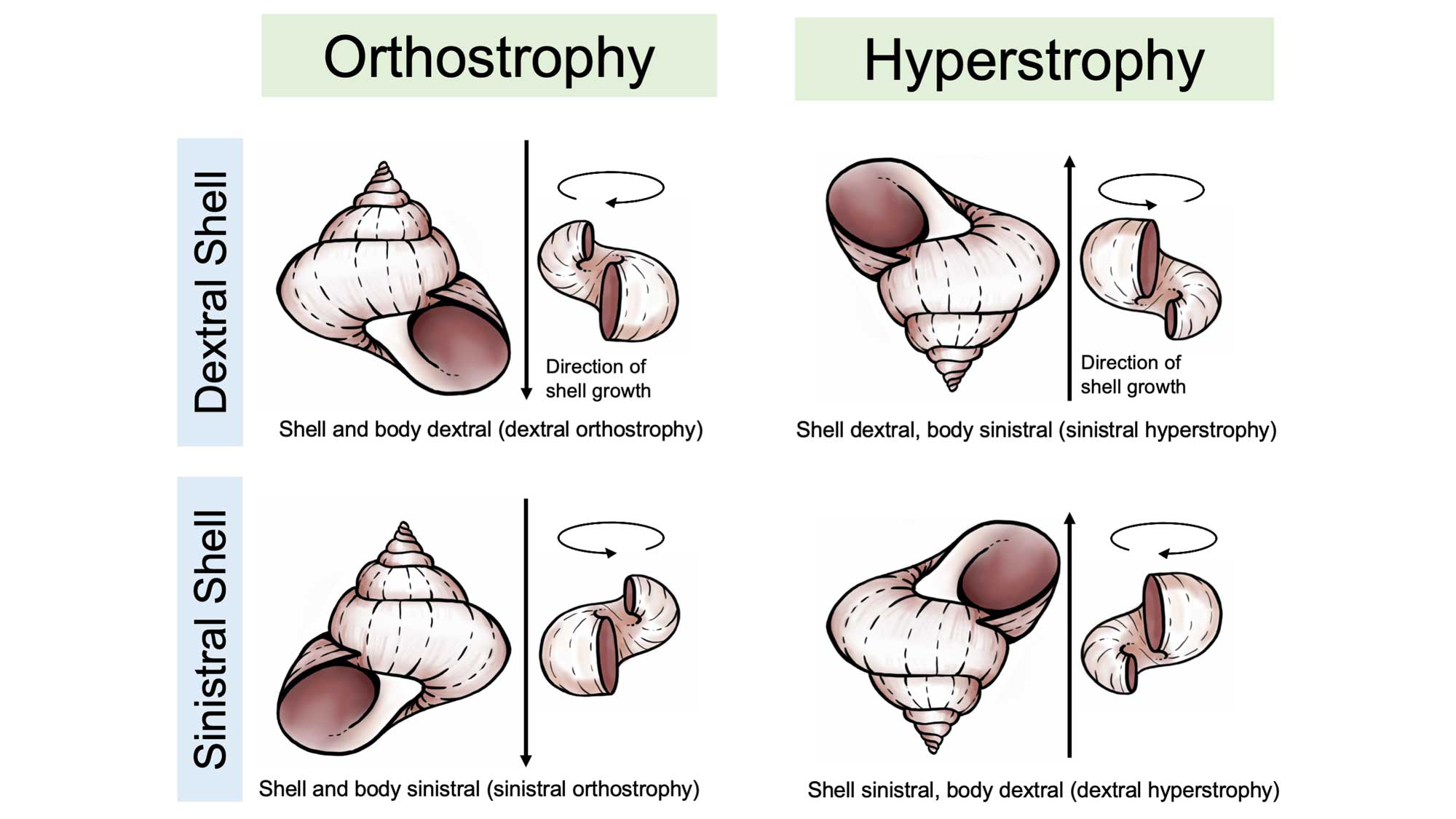
Differences between orthrostrophic and hyperstrophic gastropod shells. Image reproduced and modified from original in Ponder and Lindberg (2020); images of shells redrawn by Christi Sobel.
In some gastropods the shell coiling can be even more complex, sometimes changing chirality during ontogeny at the transition between the larval and post-larval stages. This is called heterostrophy; for example, a larval gastropod with a dextral protoconch that becomes sinistral later in development is described as exhibiting sinistral heterostrophy. Most gastropods, however, have homeostrophic shells, meaning that the larval and post-larval shells have the same chirality.

Comparison of homeostrophic and heterostrophic gastropod shells. Shell images redrawn from figure in Fryda (2021, Encyclopedia of Geology) by Christi Sobel. Bottom right image: Heterostrophic protoconch and early whorls of Pyrgiscus caribbaeus from the Miocene Cantaure Formation of Venezuela; image from pl. 68, fig. 3d in Landau et al. (2016 in Bulletins of American Paleontology; copyright Paleontological Research Institution).
Major Features of the Shell
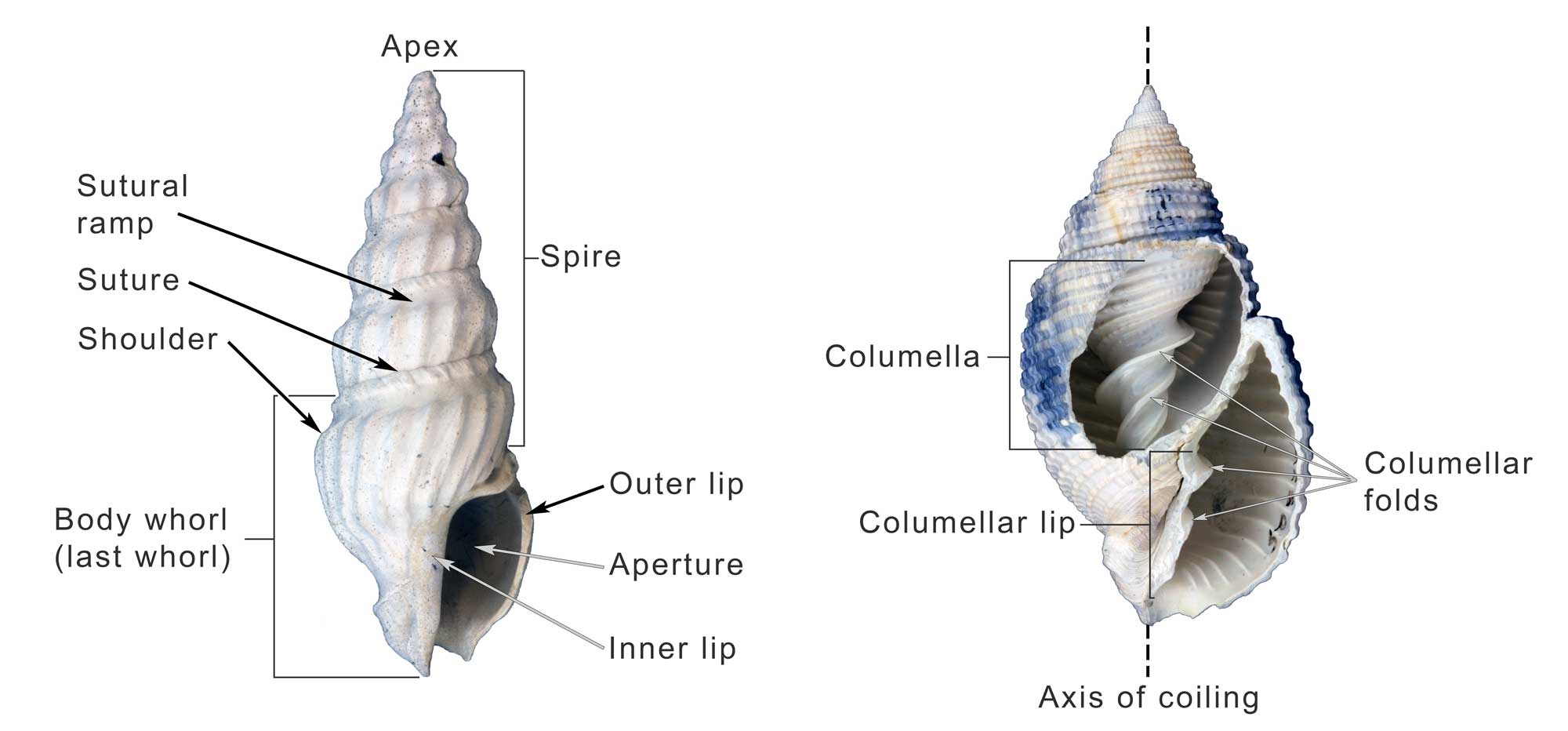
Some of the major features of the gastropod shell illustrated on Plio-Pleistocene specimens from the southeastern United States. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
Fossil specimen of the gastropod Naticarius plicatella from the Pliocene Pinecrest Beds of Sarasota County, Florida (PRI 70745), with annotations identifying major features (load model, then click on the circled numbers). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York (PRI 70745). Length of specimen is approximately 3.5 cm. Note that operculum (trap door) is remarkably preserved in situ (Sketchfab).
Anterior (siphonal) canal (notch): a tubular elongation of the anterior end of the apertural margin which, in neogastropods, holds the inhalant siphon. In some species, the siphon emerges from a notch, or indentation, in the shell rather than from a tube-like canal.
Aperture: the opening of the shell, in which the animal lives.
Apex: youngest and posterior-most part of the spire; informally called the “tip of the spire.” In especially well-preserved specimens, the larval shell, or protoconch, may be retained at the apex.
Apical whorls: the whorls that are closest to the shell apex.
Axis of coiling: an imaginary, usually straight, line around which the shell coils. This line runs from the apex of the spire (posterior end of shell) to the anterior (apertural) end of the shell.
Callus: a deposit of one of more layers of shell material on the inner lip, and sometimes more, of the shell.
Columella: an internal feature that surrounds the axis of coiling, forming a central pillar around which the rest of the shell is built.
Columellar lip: the portion of the anterior end of the shell that will later become part of the columella; it is sometimes ornamented with rib-like structures called columellar folds (also called columellar plaits), which do not have a well-established function.
Fasciole: Complex structure on the anterior end of the aperture marking successive position of the anterior siphonal notch and its associated callus.
Inner lip: the adaxial (parietal) margin of the aperture.
Last whorl (or body whorl): The anterior-most portion of the shell and the youngest whorl of the shell. Because much of the living animal’s body occupies the last whorl, this interval of growth is sometimes also called the body whorl. When startled, many snails are able to retract their bodies into the last whorl for protection.
Outer lip: the abaxial margin of the aperture, where new shell growth is deposited.
Protoconch: the larval shell of a gastropod, present when the animal first emerged from its egg. Because of their fine features, protoconchs are often absent from adult shells, owing to either erosion or breakage. They are more commonly preserved on juvenile shells.
Selenizone: spiral band of curved growth lines formed by the filling in of a notch, sinus, or slit in the outer apertural edge.
Shoulder: the anterior edge of the sutural ramp, sometimes associated with the position of maximum diameter of the last whorl.
Sinus: a notch of the aperture at the site of the exhalent current.
Slit: a thin linear cleft in the margin of the aperture, culminating at the presumed site of the exhalent current from the mantle cavity.
Spire: whorls of shell growth preceding the final half turn of the last whorl, including the protoconch and the teleoconch. The term comes from this structure’s similarity to the pointed, cone-shaped spires of cathedrals. While most gastropod species have a spire, species that do not grow downwards along their axis of coiling (i.e., do not exhibit translation) do not, as earlier whorls are covered by subsequent shell growth.
Sutural ramp: the spiral band located between the shoulder and the suture.
Suture: the continuous line on the shell surface that separate one whorl of growth from the preceding whorl, often represented by a groove.
Teleoconch: all whorls of the shell, except the protoconch. The boundary between the protoconch and teleoconch is sometimes indicated by a change in shell morphology, such as the formation of ornamentation.
Umbilicus: a depression or opening formed in association with the shell axis and located adjacent to the inner lip of the aperture.
Protoconch: The Larval Shell
The gastropod shell that begins forming during larval development is called the protoconch.

The protoconch of a fossil cone snail from the Dominican Republic viewed using a scanning electron microscope (SEM). The arrow indicates the boundary between the larval protoconch and the teleoconch, which is marked by the appearance of bumps called tubercles. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
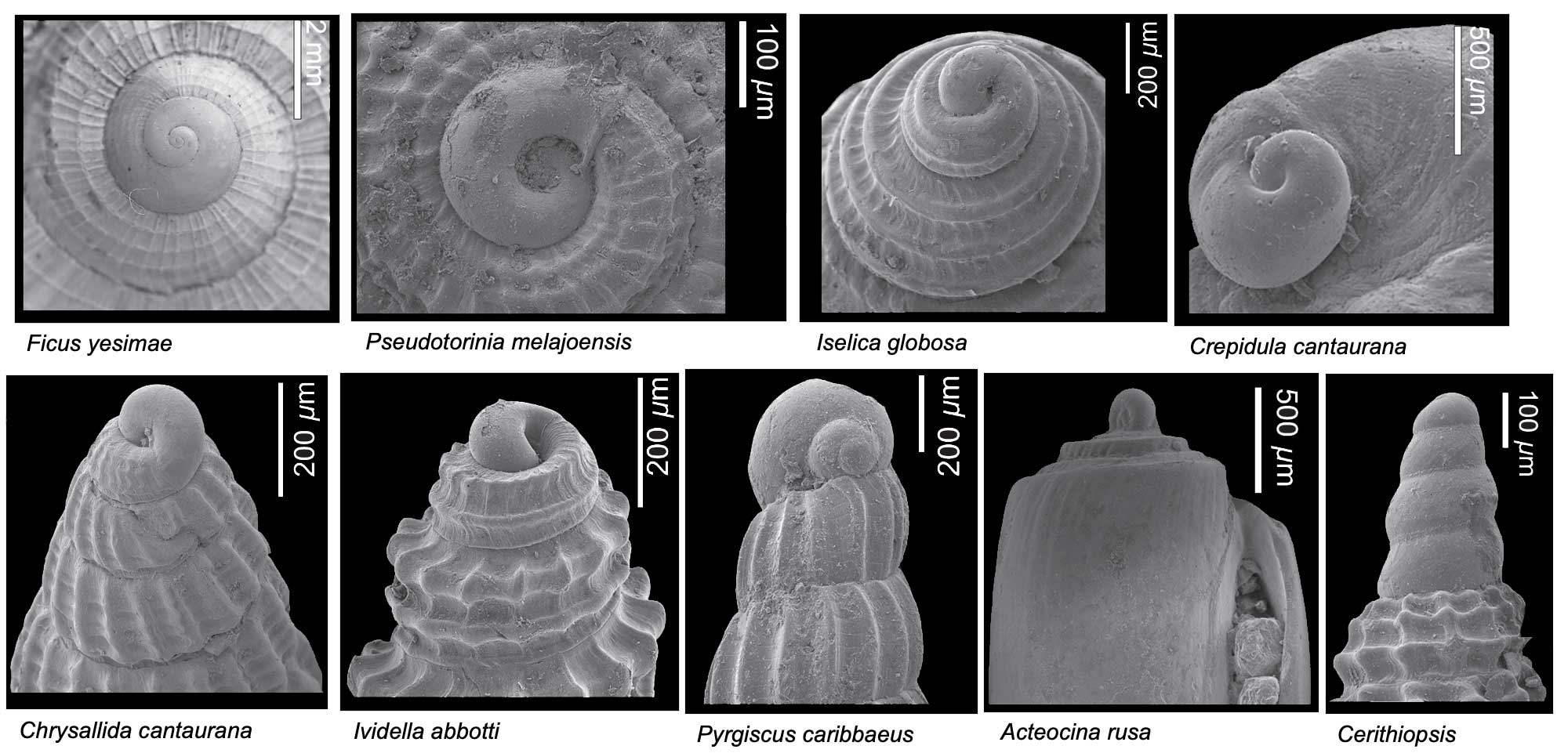
Diversity of different types of gastropod protoconchs. All specimens are from the Miocene Cantaure Formation of Venezuela. Images from Landau et al. (2016 in Bulletins of American Paleontology; copyright Paleontological Research Institution).
In marine gastropods, the form of the protoconch can be indicative of the mode of larval development. Small protoconchs with multiple whorls (called multispiral) are often indicative of species that develop and feed in the plankton (planktotrophs), whereas those that are larger and have two or fewer whorls (called paucispiral) develop mostly on the bottom and do not feed during larval development (non-planktotrophs or lecithotrophs), relying instead on a large supply of yolk. Some species have direct development and spend no time in the plankton during their development.
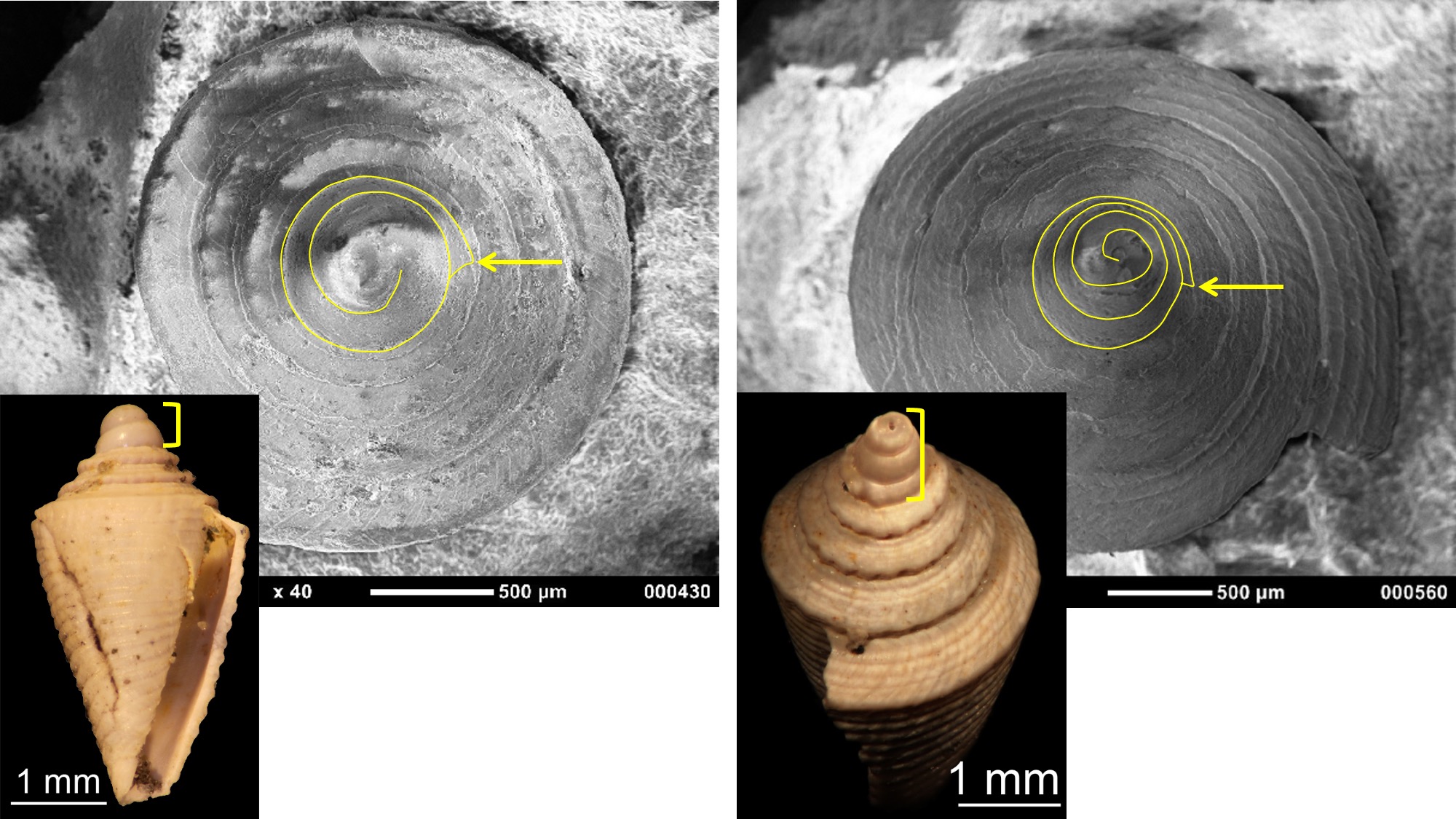
Comparison of lecithotrophic and planktotrophic gastropod protoconchs (outlined) in two Pliocene fossils from the Dominican Republic; position of protoconch-teleoconch boundary indicated by arrow. Left: Conus symmetricus has a protoconch with about 2 whorls and a diameter of about 0.7 mm, indicative of lecithotrophic development. Right: Conus planiliratus has a protoconch with over three whorls and a diameter of about 0.7 mm, indicative of planktotrophic development (the earliest protoconch whorl(s) are missing in the specimen shown). Specimens are from the collection of the Paleontological Research Institution. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
The protoconch of a planktotrophic gastropod consists of an embryonic shell (called protoconch I) and a larval shell (called protoconch II). Following metamorphosis, the adult-portion of the shell (called the teleoconch) begins to form. The embryonic shell usually consists of about one coil or whorl and shows no accretionary growth because it is entirely formed by the shell gland. Its size reflects the amounts of yolk. The multispiral larval shells of gastropods with planktotrophic development may exhibit growth lines, reflecting their accretionary growth during the larval stage.
At metamorphosis, a distinct change in shell morphology–from the protoconch to the teleoconch–can often be recognized, and is indicated by a change in ornamentation (e.g., see image above). Being able to identify this transition allows a biologist or paleontologist to characterize the size and number of whorls of the protoconch, which in turn allows one to predict the developmental mode of the snail (i.e., planktotrophic vs. non-planktotrophic), even in specimens that are hundreds of millions of years old.
Shell Microstructure and Color
Gastropod shells are composed of a combination of calcium carbonate crystals (calcite and/or aragonite) and organic molecules. The shell grows by accretion at the margin of the aperture and internal deposition, both performed by the mantle. This step-by-step process is often recorded by axial growth lines on gastropod shells. Growth lines are formed episodically and do not correlate with a standard amount of lapsed time (unlike, for example, tree rings, which mark one year of growth). The interior of the shell can also be “remodeled” by dissolution by the animal. This is a hallmark of cone snails, which remodel the interior of their shells as they grow (see Kohn et al., 1979 for an illustration). The bubble snail Haminoea also resorbs earlier whorls.
The gastropod shell often consists of three or four layers:
- Periostracum: An organic outermost layer, which may be transparent, translucent, or dark and opaque. It is the first part of the shell to be secreted by the snail as the shell grows. After a snail dies, the periostracum quickly peels away from the shell.
- Prismatic layer: Shell layer composed of either calcite or aragonite. The crystals may be elongate and oriented perpendicular to the shell surface (also known as columnar), or may be granular and indistinctive in shape. The prismatic layer is typically the outermost shell layer.
- Cross-lamellar layer: Shell layer composed of aragonite. Crystals within the layer are inclined at different angles, giving a distinctive appearance. When present, it is often a middle shell layer; absent in some groups.
- Nacreous layer: Iridescent shell layer composed of aragonite, also known as "mother-of-pearl." Crystals form thin, plate-like laminae. It forms the innermost shell layer in some snails (e.g., Vetigastropoda).
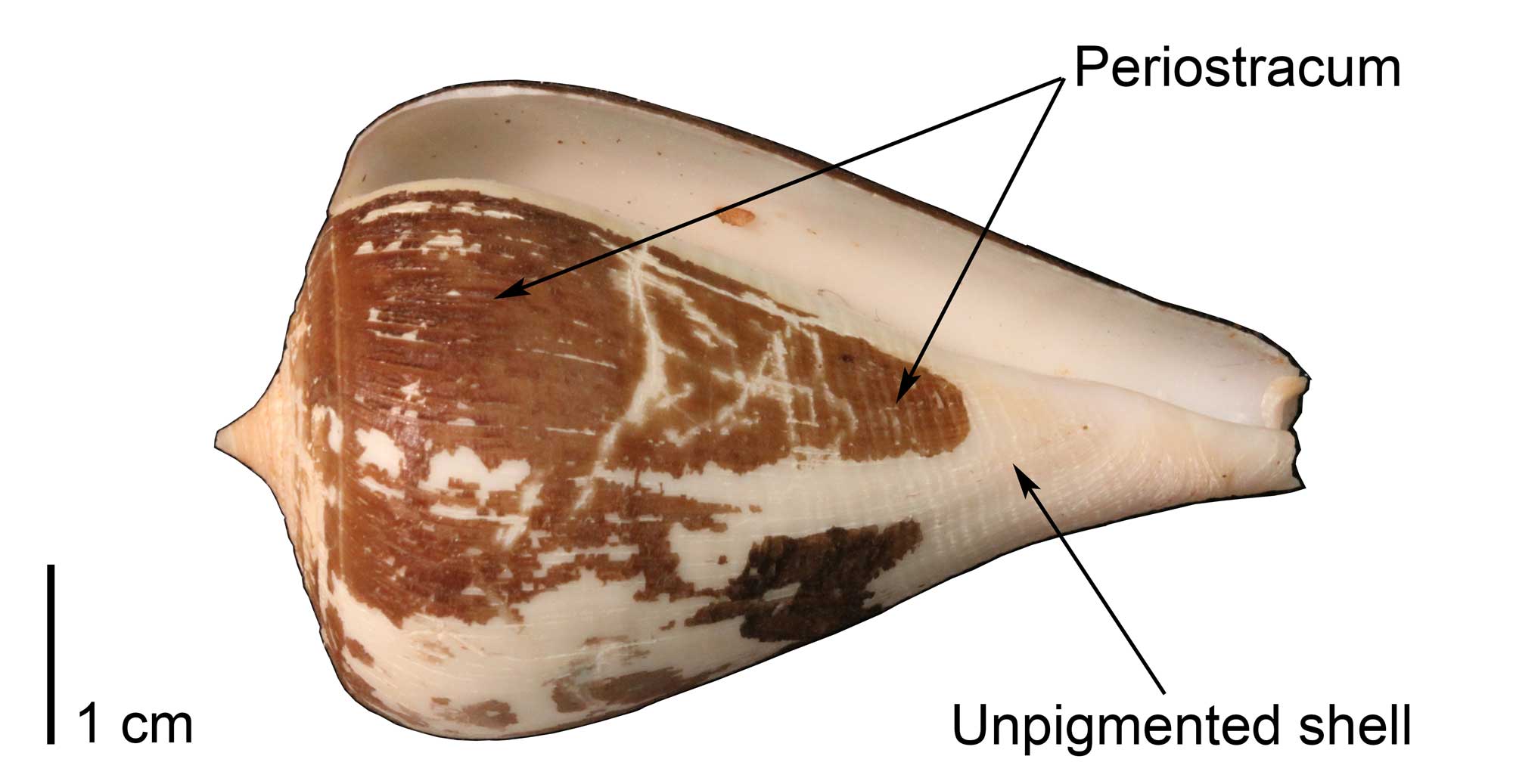
Specimen of the extant cone snail Conus patricius, with portions of the organic, brown-colored periostracum preserved (UF Malacology 321278). Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
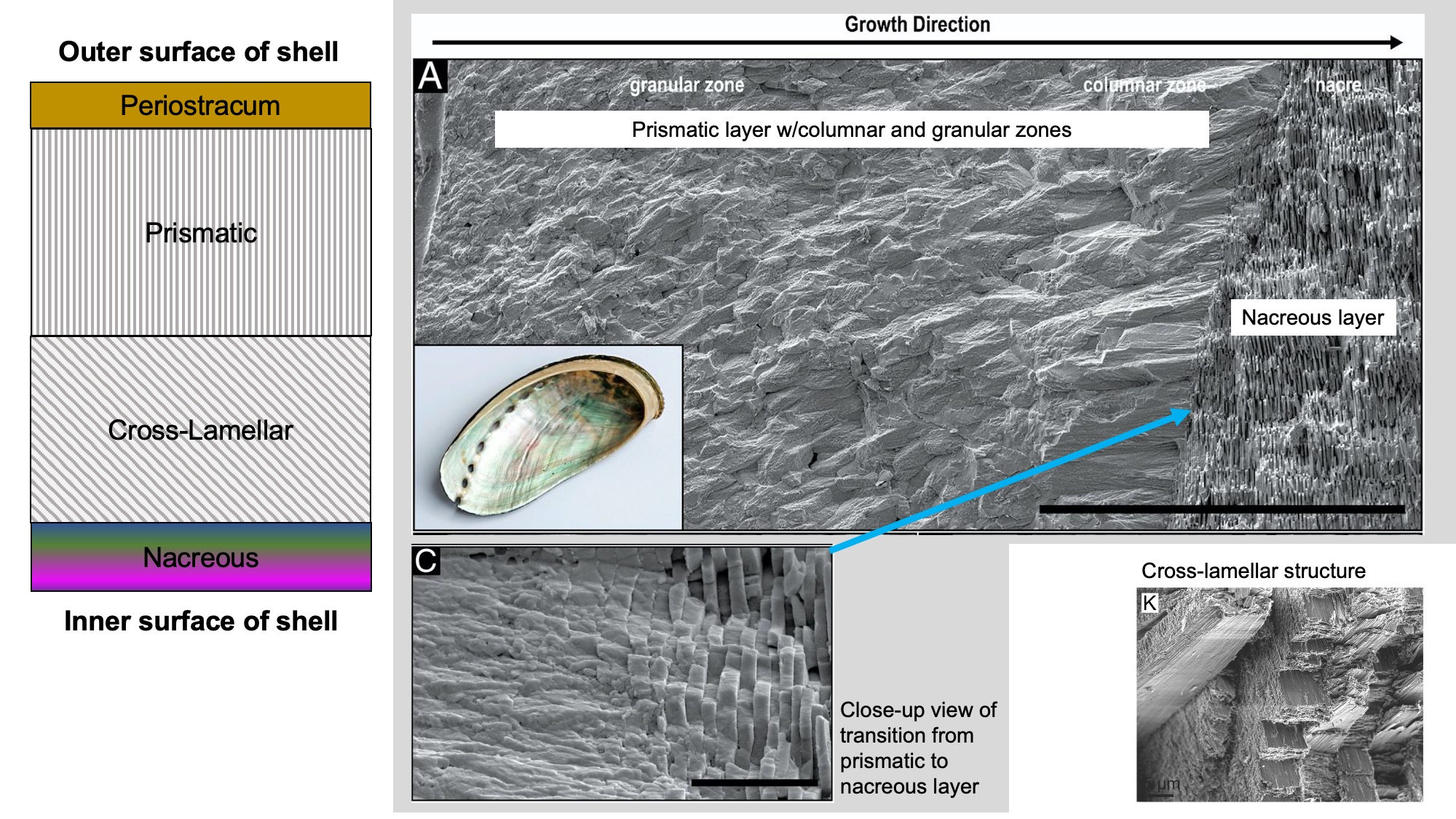
Gastropod shell microstructure. Left: Common ordering of microstructural elements in gastropod shells. Top right: SEM image of a cross section of abalone shell (A), showing the transition from the prismatic layer to the nacreous layer, with close-up view of this transition (C). Images from fig. 5a, 5c in Schoeppler et al. (2019 in PNAS; Creative Commons Attribution 4.0 International license 4.0). Bottom right: Cross-lamellar structure in Semicassis granulata (K). Image from fig. 1k in Checa (2018 in Frontiers in Marine Science; Creative Commons Attribution 4.0 International license).
There are many different arrangements of these layers in different gastropod groups, as well as a variety of different types of microstructures. Patellogastropods have more diversity in shell layers than Caenogastropods, which have simpler multi-layered shells formed of cross-lamellar microstructures. Iridescent nacre (also known as mother-of-pearl) is present only in Vetigastropods like abalone (Haliotidae).
Modern specimen of the abalone gastropod Haliotis rufescens from California (PRI 70105); the interior of the shell shows the iridescent nacre that is characteristic of vetigastropods. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of specimen is approximately 16.5 cm.
The shells of gastropods and other mollusks are frequently decorated with coloration patterns that have made them objects of human interest for thousands of years.

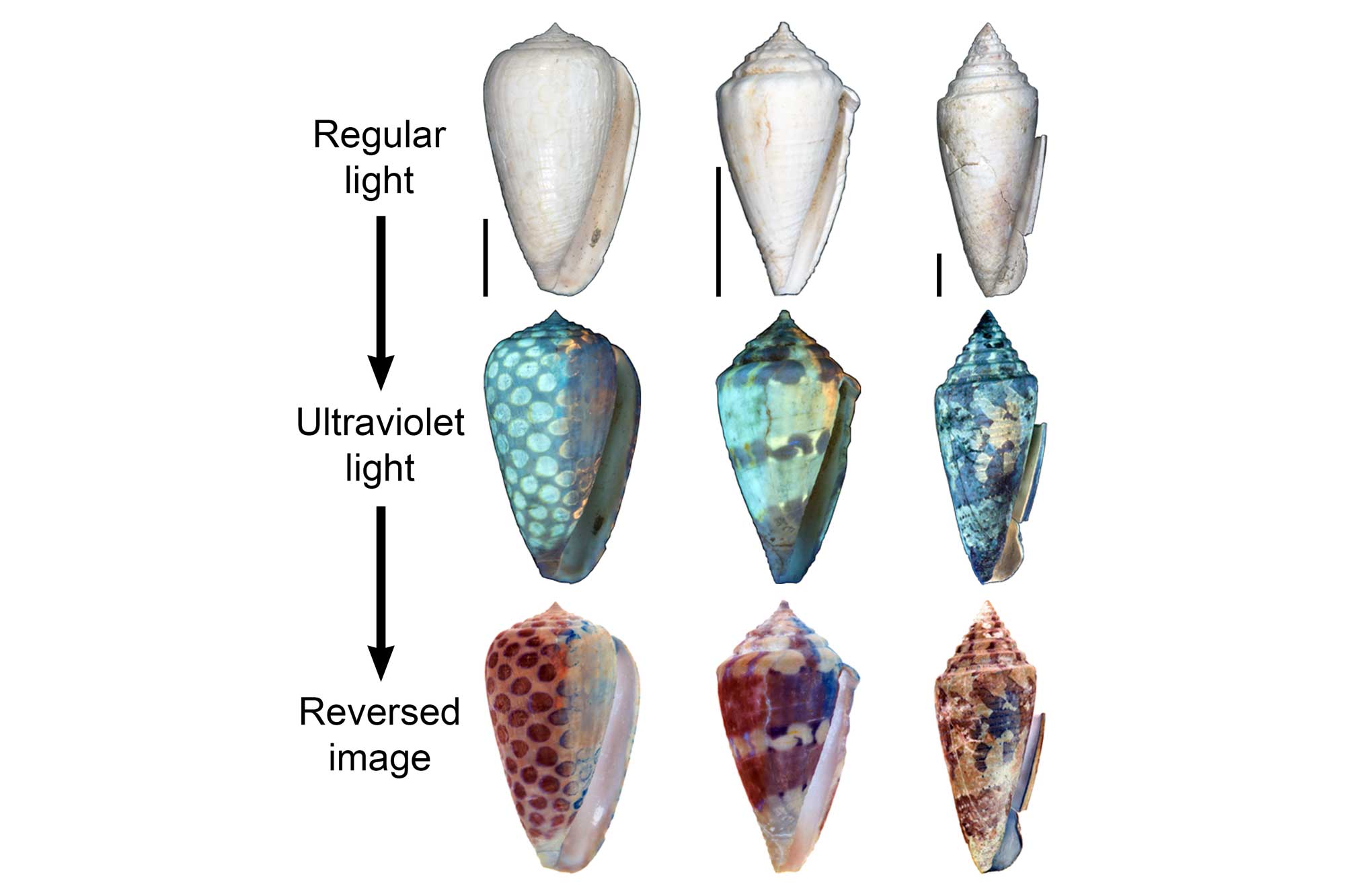
These patterns are formed by different organic pigments that are laid down in the uppermost calcified layer as the shell is built. Coloration patterns are occasionally preserved in fossils as old as the Devonian. Regions of original pigmentation sometimes fluoresce when exposed to ultraviolet (UV) light, revealing the coloration patterns of long-extinct species, if not the colors of the pigments themselves.
Functional Morphology of the Gastropod Shell
Functional morphology is the study of the relationship between the form of a body part and how it is used by an organism in its environment. It is the study of what a structure is "for"; for example, legs are for walking, jaws are for biting, and wings are for flying. The shapes of gastropod shells are, in general, less directly related to function and mode of life than the shells of bivalves. This is mostly because the shells of bivalves are frequently in direct and regular contact with the substrate (i.e., the seafloor) and so their form needs to be suited to deal with living in and on the bottom, whereas gastropod shells are frequently carried above the substrate. Nevertheless, a number of features of gastropod shells are clearly related to their mode of life and where they live. Features such as spire height, and apertural inclination and shape can strongly affect how easily snails can balance and move their shells. Corresponding "Laws of Gastropod shell form" were formulated by Robert Linsley (1977):
- The law of radial apertures: “Gastropods of more than one volution with radial apertures do not live with the plane of the aperture parallel to the substrate. Most typically, it is perpendicular to the substrate.”
- The law of tangential apertures: “Gastropods of more than one volution with tangential apertures live with the plane of the aperture parallel to the substrate.”
- The law of shell balance: “If the shell of a gastropod is supported above the body, it will be positioned so that the center of mass of the shell and its contents is over the midline of the cephalopedal mass.”
- Law of apertural re-entrants: “Angulations or re-entrants on the aperture indicate inhalant or exhalent areas; inhalant areas will be directed anteriorly.”
- Law of apertural elongation: “Gastropods having elongated apertures possess only a single gill and develop a water flow through the mantle cavity from anterior to posterior, along the long axis of the aperture; this axis is subparallel to the anterior-posterior axis of the foot.”
The cap-like “limpet” shell shape is clearly well-suited for clamping securely onto hard substrate, and, largely for this reason, it has evolved independently multiple times over the past 500 million years from coiled ancestors.
Very high-spired living gastropods, such as terebrids and turritellids, can withdraw their bodies far into their shells, and this may explain why this shell form has evolved numerous times.

A dense accumulate of fossil Turritella shells in the Miocene Gatun Fm. of Panama. Photograph by Jonathan R. Hendricks.
A number of features of gastropod shells appear to function to resist predators, including smooth and slippery surfaces, greater thickness, thickened outer lips, narrowed or obstructed apertures, and sculptural elements such as knobs, spines, and spiral ribs.
Gastropods with elaborate spines and thickened shells are especially common in tropical marine habitats that are occupied by shell-crushing predators like crabs. In contrast, the shells of freshwater and terrestrial gastropods tend to be much smoother and have fewer ornaments, which may require significant energy to build. The mineral resources necessary to build shells are also often more limited in freshwater and terrestrial habitats than they are in the ocean. Even though their shells may be of limited utility against some predators, the shells of freshwater and terrestrial snails provide a very important benefit: they help the snail to resist drying out.

The brown tree snail Amphidromus inversus, which has a smooth shell. Photograph by "budak" (Flickr; Creative Commons Attribution-Non-Commercial-NoDerivs 2.0 Generic license).
Other features of shells also help snails to avoid predators. Some marine snails (e.g., Conus, Bulla) have smooth shells that are streamlined for burrowing. Others like Xenophora (carrier shells) take the opposite approach and cement the shells of other mollusks to their own, providing camouflage and a difficult surface for predators to grasp.

Two specimens of modern Xenophora, or carrier snails. Upper left: Top (dorsal) view of Xenophora calculiferus from China (PRI 100304). Bottom left: Top (dorsal) view of Xenophora robusta from Guaymas, Mexico; right two images are magnified views of the sides of the same specimen. Specimens are from the collections of the Paleontological Research Institution, Ithaca, New York. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
The color and coloration patterns of gastropod shells may also be functional in some cases, for example providing camouflage from predators. In other cases, coloration patterns may not have any function at all. Other features of shell sculpture, such as axial ribs and spiral cords, may function to assist in burrowing in soft sediment. Whatever the functional importance of their external form, gastropods shells have clearly been subject to widespread and repeated convergent evolution (homoplasy).
As is the rule in nature, there are of course some exceptions to the "laws" of gastropod shell form outlined above. While almost all gastropod shells consist of one valve (and are therefore referred to as “univalve”), a few are bivalved. And while gastropod shells are usually said to differ from cephalopod shells in lacking septa, many actually possess them, if only in the very early stages of shell growth.
"Bivalved" gastropods
Gastropods of the heterobranch family Juliidae (e.g., Berthelinea) live in tropical oceans around the world, and have a two-part, clam-like shell. Species in this family hatch from their eggs as swimming larvae with typical coiled, univalved shells. After metamorphosis, the shell splits to form a second valve, complete with a functional hinge; the left valve retains the distinctly gastropod-like protoconch. The animal is able to fully retract into its two valves, which provide protection. Also similar to bivalves, these snails also produce a mucous tether to attach to the substrate that functions like the byssus of a bivalve. These snails have a fossil record that begins in the Paleocene. Their bivalved shells were mistaken for those of bivalves until the middle of the 20th century (LeRenard et al., 1996).

Two specimens (one large, one small, both bright green) of the bivalved gastropod Berthelinia singaporensis (Family Juliidae) on the alga Caulerpa peltata. Photograph by "budak" (Flickr; Creative Commons Attribution-NonCommercial-NoDerivs 2.0 Generic license; image size slightly increased).
Septa in Gastropoda
Although septa (walls of shell material that leave spaces sealed off from the body cavity) are frequently said to be characteristic of cephalopod shells, and to be absent or rare in the shells of gastropods, septation occurs in many gastropod groups, especially in the family Turritellidae and other caenogastropods, as well as some shelled heterobranchs (e.g., Architectonicidae) and some Paleozoic forms (e.g., Euomphaloidea, Murchisonioidea, and Loxonematoidea), albeit mainly in the early whorls of the shell. Unlike the situation in cephalopods, septa in gastropods do not appear to have an obvious function, and may be structural side-consequences of secondary internal shell thickening (learn more about such “spandrels” here).
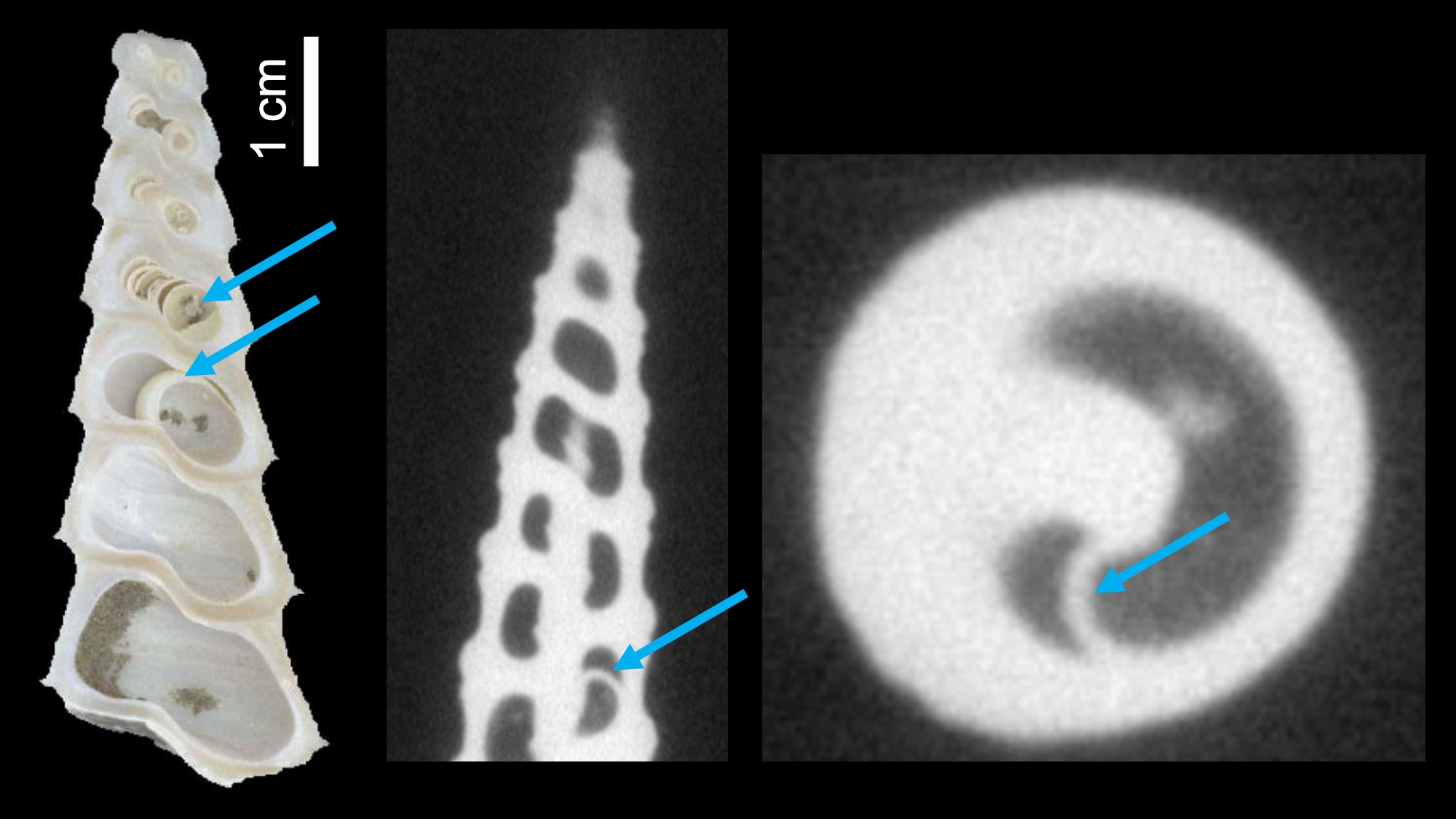
Examples of septa in the high-spired shells of Turritella. Left: Septa in a sectioned Miocene specimen of Turritella abrupta. Right two images: An example of a septum inside the shell of Terebra subulata. Image captured using CT scanning and provided courtesy of Brendan Anderson; see also Anderson and Allmon (2018 in Paleobiology).
Gastropod Development and Growth
Torsion
One of the great mysteries of gastropod evolution is torsion. Initially, the mantle cavity (which holds the gills and sensory and excretory organs) and anus are positioned at the posterior end of the animal. Later in development, these structures are rotated counterclockwise up to 180 degrees, placing the mantle cavity and the organs it houses–as well as the anus–right above the animal’s head. Further, torsion causes the gut to become U-shaped and important nerves to cross over one another. Why gastropods undergo torsion remains unclear, but they all do it.
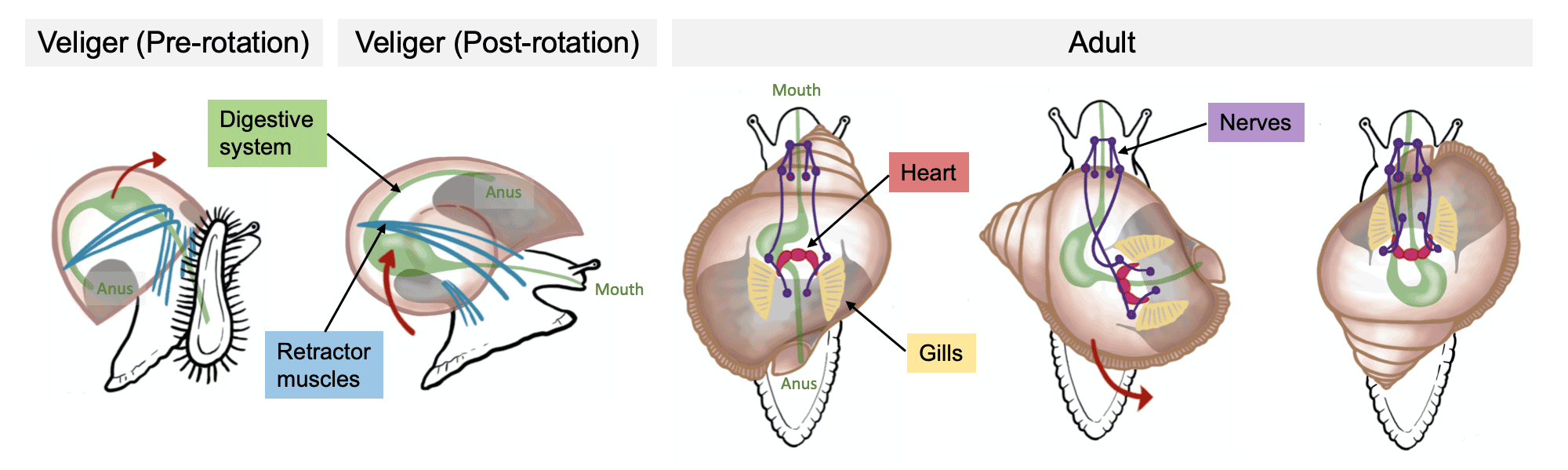
The process of torsion in gastropods. Images of snails redrawn from Ponder et al. (2020, Biology and Evolution of the Mollusca) by Christi Sobel.
To complicate matters, some gastropods (e.g., opisthobranchs) undergo torsion early in development, but then the process seemingly reverses itself back to nearly the starting condition later in development. This is called detorsion and is a result of differential growth caused by one side of the animal's body growing faster than the other (not actual untwisting). Importantly, torsion is not directly related to the coiling of the shell, and the foot of the animal is unaffected by the process.
Larval Development in Marine Species
The initial stage of the shell that forms within the egg is called the embryonic shell. It forms part of the protoconch (see above). Once they hatch, embryonic gastropods may live on the seafloor as juveniles that resemble the adults (direct development). Other species may float as larvae in the plankton and are called veligers.
"What is a Veliger" by CollinLabPanama (YouTube). This video shows larval development of a calyptraeid gastropod Crepidula or Slipper Limpet.
Veliger larvae may either feed on plankton (planktotrophic) or be non-feeding (nonplanktotrophic or lecithotrophic). As noted above, the protoconchs of direct developers and nonplanktotrophic larvae are typically larger and have fewer whorls than those of planktotrophic species. Compared with nonplanktotrophic species, taxa with planktotrophic larvae tend to spend longer amounts of time in the plankton feeding prior to metamorphosis. As a consequence, planktotrophic species have greater geographic distributions than nonplanktotrophic species, as they may be transported great distances by currents before settling.
Differing geographical ranges of three closely-related species of modern cone snails. Conus marmoreus and Conus bandanus have planktotrophic development, while Conus araneosus has nonplanktotrophic development. Image source: fig. 1 in Hendricks (2012 in Evolution: Education and Outreach; Creative Commons Attribution 2.0 International license).
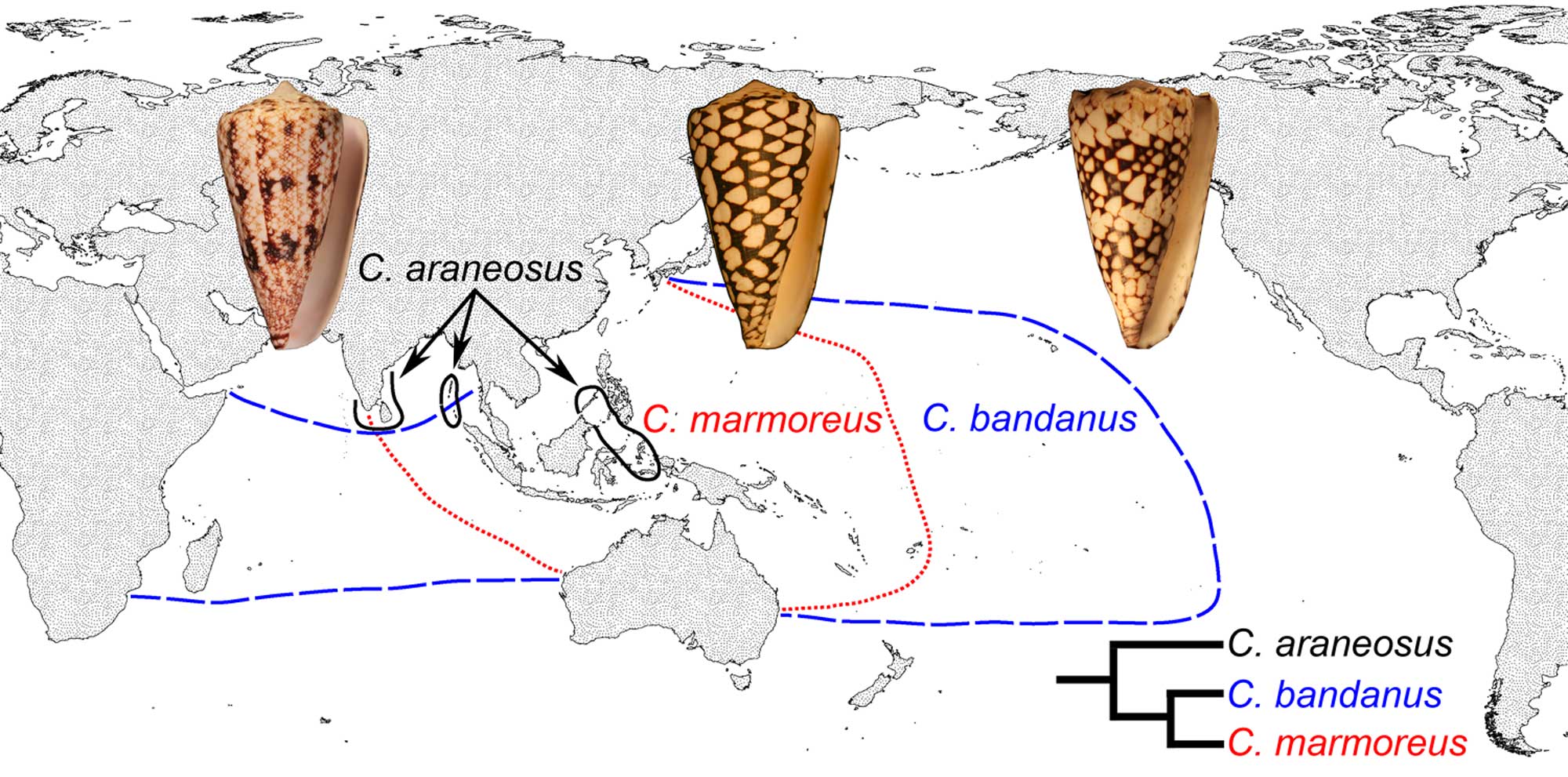
The earliest gastropods were probably nonplanktotrophic. Planktotrophic larval development originated late in the Paleozoic and is absent in the most basal gastropod clades (e.g., Vetigastropoda). Variation in developmental mode characterizes some derived gastropod groups, with even closely-related species exhibiting either planktotrophic or nonplanktotophic larval development (see cone snail example above). Rarely, there can be variation in developmental pattern within a single species, a condition called poecilogony.
Being able to differentiate planktotrophic from non-planktotropic species has allowed paleontologists to address questions such as whether developmental characteristics correlate with geographic range and therefore extinction resistance, as far-flung, widely-ranging species are predicted to be less likely to go extinct than species with small ranges. Learn more in the Macroevolution chapter of the DEAL.
Adult Shell Growth
The coiled gastropod shell is a conical tube wrapped in a spiral around a central, usually vertical, axis. The shell gets larger by adding material at the opening. From this simple plan, gastropods have evolved an enormous array of shapes by varying different aspects of their growth. The shell may expand rapidly, or more slowly. The spiral may be tightly or loosely coiled. It may be coiled in a plane or it may form a helix, coiling in a third dimension along the axis. The opening of the shell may have a wide variety of shapes, from a circle to a narrow slit, and be oriented in various attitudes relative to the coiling axis. The outer surface of the shell may be smooth or be ornamented with a wide variety of spines, knobs, vertical ridges, or spiral ribs. The shell surface is usually a solid wall, but it may also have holes or slits. Biologists and paleontologists have developed a variety of terminology and models to encompass this great diversity of form.
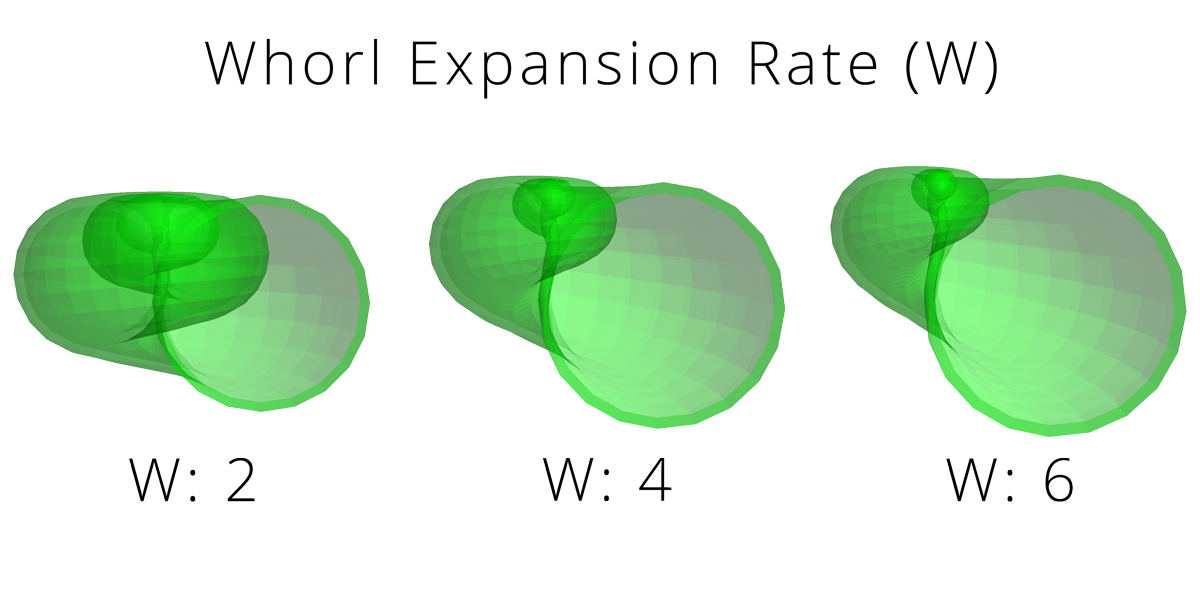
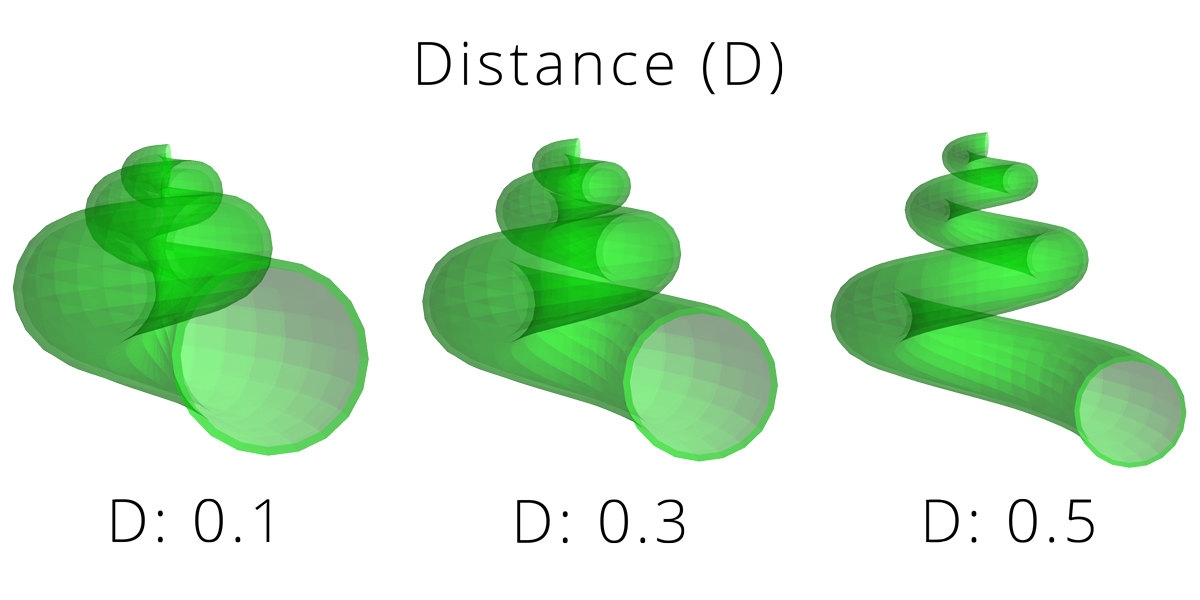
One of the most widely used models was proposed by paleontologist David Raup in the 1960s. Raup suggested that the overall shapes of coiled shells (including gastropods) can be modeled by a few simple parameters:
Rate of whorl expansion (W): measurement of how rapidly the size of the generating curve (i.e., the aperture) expands.
Rate of translation (T): measurement of how rapidly (or if) the shell grows down the axis of coiling.

Distance of generating curve from the axis of coiling (D): measurement of how tightly coiled a shell is. “Unwound” gastropod (and ammonite cephalopod) shells have high values of D.
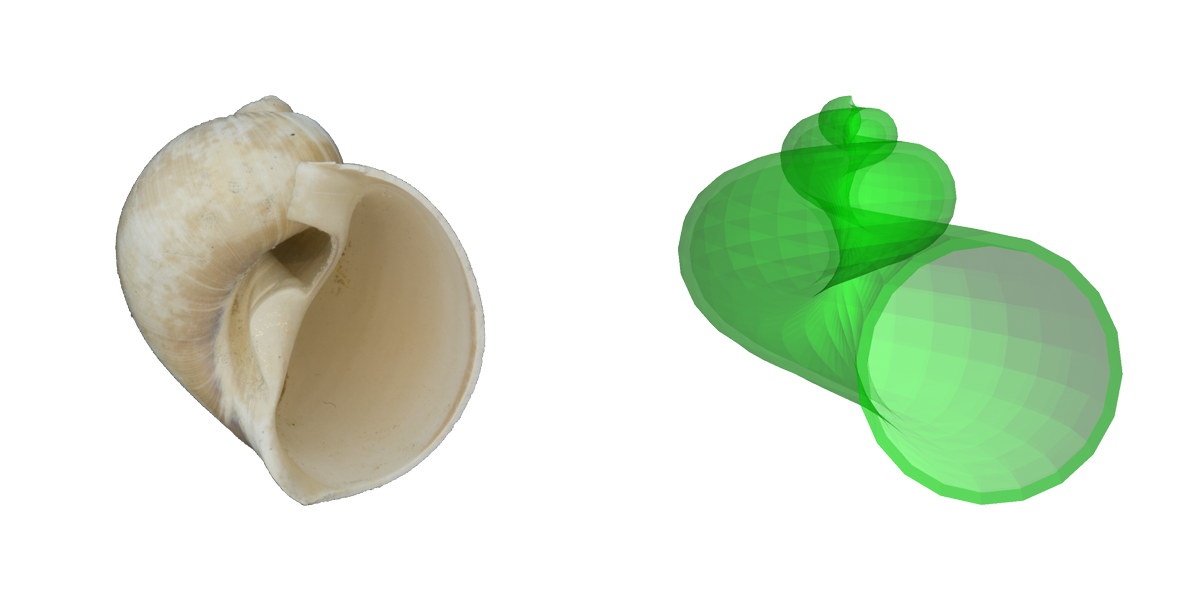
Comparisons of a real shell (fossil naticid, or moon snail) and a simulated shell based on Raup's parameters. Image by Jonathan R. Hendricks for the Digital Atlas of Ancient Life project.
Resman et al. (2011) recently developed a software tool for modeling gastropod shell form based on Raup’s three parameters. This program–called “Shell Parameter Space” can be downloaded. Note that using this program requires first downloading the free software Wolfram Demonstrations Project. The simulated gastropod shells shown above were generated using this program.
Modifying the three Raup parameters (W, D, and T) in combination results in many shell forms that approximate those of fossil and modern gastropods. Very interestingly, Raup (1966) found that many theoretically possible shell forms have never been realized in nature. The reasons why this is true are fascinating to consider and range from architectural constraints on form to regions of morphospace that have never been “explored” in the history of mollusk evolution.
"Functional Morphology Lecture II" by Phoebe Cohen (YouTube).
Gastropod Shell Sclerochronology
Fossil and modern gastropod shells can yield information about their chronological age at death through the methods of sclerochronology. This technique uses the temperature-dependence of the ratio between two stable (i.e., non-radioactive) isotopes of oxygen when it is incorporated into the calcium carbonate (CaCO3) of the gastropod shell. CaCO3 precipitated in warmer water is relatively high in 16O and depleted in 18O (“isotopically light”); CaCO3 precipitated in cooler water is relatively high in 18O and depleted in 16O (“isotopically heavy”). By taking samples across the shell, a profile of isotopic measurements can determine how many annual temperature cycles the snail lived through, and therefore how many years old it was when it died. This allows investigation of a wide variety of topics in extinct species, from growth rates to heterochrony in evolution.
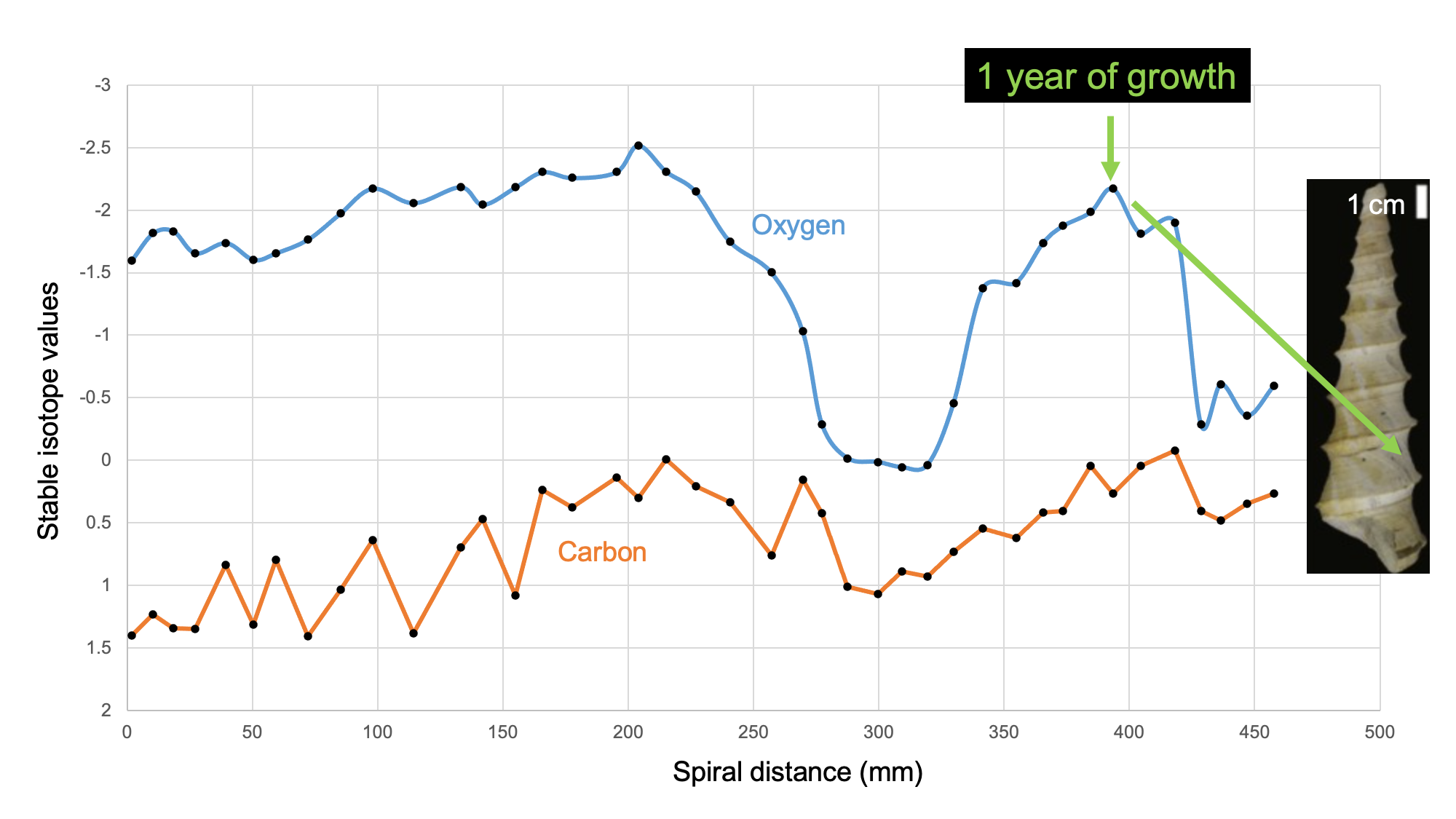
Learn more about biogeochemical analysis of fossil samples in the Paleoecology chapter of the Digital Encyclopedia of Ancient Life.
Content usage
Usage of text and images created for DEAL: Text on this page was written by Warren D. Allmon or Jonathan R. Hendricks. Original written content created by Warren D. Allmon or Jonathan R. Hendricks for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by Jonathan R. Hendricks or Warren D. Allmon are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.




