Chapter contents:
Evolution and the Fossil Record
– 1. Natural selection
– 2. Species and species concepts
– 3. Speciation
– 4. Punctuated equilibria and stasis
–– 4.1 Videos about punctuated equilibrium and stasis
– 5. Macroevolution
–– 5.1 Hierarchies
–– 5.2 Species selection
–– 5.3 Abiotic vs. biotic causes of macroevolution ←
–– 5.4 Evolutionary radiations
Darwin, competition, and the Modern Synthesis
When Charles Darwin parsed out the relative contributions of the physical environment and competition to evolution, he held that competition among and within species was a much more prominent factor than the physical environment. His perspective fits into a broader historical perspective on the topic. For instance, Augustin de Candolle—the noted early 19th century botanist whose views very much influenced Darwin—posited that “all nature was at war with itself.” This perspective was also influenced by the cultural milieu of the time. For example, an important view on economics and societies in England during the Victorian era was Adam Smith’s (1776) “Wealth of Nations,” which asserted that those nations that allowed businesses to freely compete in the marketplace would ultimately develop the most effective businesses; this would in turn produce the best nations. On the biological side, Darwin held that those species that faced the most competition (and survived) would become the most fit (or, well adapated) and ultimately succeed relative to other species. He applied this framework to many phenomena, including what he saw as the success of North American faunas relative to those in South America when the two were introduced to each other during the Great American Biotic Interchange. Darwin's views on competition fit neatly with then-prevalent ideas on culturalism imperialism (ideas that are now rightly discredited), though we should not assume that Darwin himself intended that his views be applied in this manner.
This emphasis on competition as the primary (albeit not sole) driver of large-scale evolution, relative to the physical environment, was largely carried forward by the principal architects of the Modern Synthesis. George Simpson, for example, very much emphasized that competition was of paramount importance in determining major changes in vertebrates diversity over time. Climate change could be a factor that initiated evolutionary change, but a major emphasis was how it bred mammals that were more competitively fit, and thereby apt to succeed against other, less fit lineages. Simpson’s perspective related to the tradition espoused by many paleontologists that preceded him. For instance, his mentor William Diller Matthew argued that climate played a role in shaping evolution—with his paradigm example involving horses—but it fit into the competition-driven framework elucidated by Darwin: northern environments created “hardier” organisms that were more competitively fit; as these hardier organisms moved into other regions, they would encounter less-fit organisms in southerly latitudes and drive them to extinction.
Perhaps the ultimate exposition of the Neo-Darwinian perspective on this topic is Van Valen’s (1973) key and highly cited paper that introduces his Red Queen hypothesis.
The Red Queen Hypothesis
In 1973, University of Chicago evolutionary biologist Leigh Van Valen published a paper—in his own "in-house" journal, Evolutionary Theory—that presented what he characterized as "A New Evolutionary Law." In many respects, this paper represents the logical culmination of theoretical ideas on the prevalence of competition in nature, which emphasized gradual, within-species transformation. Van Valen used data on the geological durations, or "lifespans" of 25,000 taxonomic groups through time to argue that extinction rates have remained constant over very long intervals across many different groups. He did this by determining the diversity of each group and how long its subtaxa persisted over time. For example, Van Valen analyzed the survivorship of extinct families of brachiopods over time, measured by how many 10's of millions of years each family survived. One-hundred and eighty (180) brachiopod families survived at least 10 million years, but of those, only four survived for over 250 million years. This is shown in the set of graphs on the left below (including Van Valen's original chart; his data replotted; and his data transformed from a logarithmic to a linear Y-axis). The plots on the right show a similar pattern for a very different group: extinct genera of mammals.
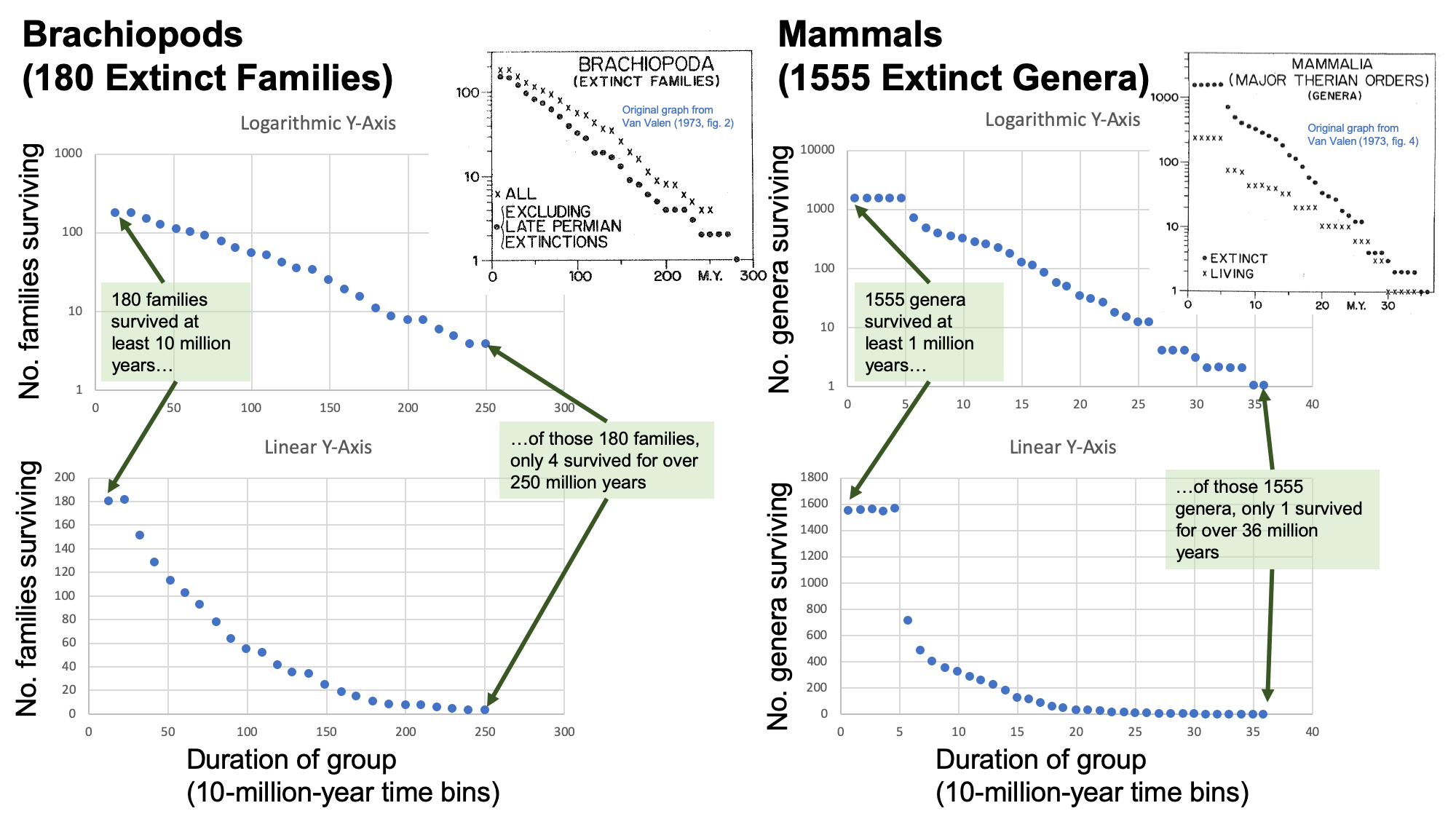
Two examples of the kind of data published by Van Valen (1973) in support of his argument for the Red Queen Hypothesis (left, extinct brachipod families; right, extinct mammal genera). Van Valen's original plots are shown, as are reproduced data from his charts shown with both logarithmic (top) and linear (bottom) y-axes. Note that the x-axis shows duration across 10 million year time bins; it does not show a geological time scale. Image by Jonathan R. Hendricks.
Note the strongly linear trends in both the brachiopod and mammal datasets when plotted against a logarithmically transformed y-axis. Van Valen argued that such trends supported a constant rate of extinction within major groups of organisms (though his data showed that this rate, given by the slope, varies across groups). This observation suggested to him that the "probability of extinction of a taxon is then effectively independent of its age" (p. 17); this means that having been around a while does not provide a given genus with any innate protection against the likelihood of extinction.
Van Valen termed this a “law of constant extinction.” Further, Van Valen argued that groups were continually in competition with one another and therefore continually adapting to one other, such that no group truly ever advanced or became “better” relative to any other group. Based on a subsection of the original paper, this has come to be known as "The Red Queen Hypothesis," named for the Red Queen in Lewis Carroll's (1871) book, Through the Looking Glass. The Red Queen was always running forward, but never getting anywhere; she states, "Now here, you see, it takes all the running you can do, to keep in the same place." Van Valen said the same is true of species: they compete, but evolution is a zero-sum game and the success of one species must be balanced by the detriment of another because resources are limited in nature.

Illustration of Alice and the Red Queen by John Tenniel in the chapter "The Garden of Live Flowers" in Through the Looking Glass by Lewis Carroll (1870); image from Wikimedia Commons (public domain).
While the Red Queen Hypothesis is a fascinating idea that has garnered significant interest in the fields of evolutionary biology and paleobiology, it suffers from several problems that suggest it might not be valid. First, it seems that Van Valen misinterpreted his data. Current evidence shows that extinction rates have not been constant through time. In fact, they have varied tremendously throughout the history of life and this is best exemplified by the existence of mass extinctions, which we shall describe in a subsequent chapter of this textbook.
Second, there is no evidence that different species—not to mention genera or families (the taxonomic levels Van Valen focused on)—are in direct competition with one another, such that one group drives another to extinction. By contrast, to build on the hierarchical perspective that we have already discussed, different populations in different ecosystems interact with one another. Further, the nature of these interactions is not consistent across each separate ecosystem. Instead, different populations within a single species experience differing selection pressures depending upon where they live. Contrary to the message of the Red Queen Hypothesis, species are not "running in place" over geological time because of competition, but rather are constant throughout much of their existence due to the processes we already discussed earlier in this chapter in relation to punctuated equilibria.
Note that we are not arguing that competition is not important; of course it is. In fact, competition for resources is one of the ways that ecologists can recognize the interactions that partly define ecosystems and communities. But does this lead to species continually adapting, and “new adversaries grinningly replacing losers” as one species drives another to extinction, as Van Valen so mellifluously phrased it? We don’t think so. Most times species do not interact and are more like what Gould and Calloway (1980) termed “ships that pass in the night." Sometimes of course, populations and organisms of different species do interact, but even then, we suspect the situation will be akin to the story of African lions and hyenas. Their interactions have been beautifully documented in a variety of spectacular, albeit sometimes bloody, nature films.
"Lion Attacked by Clan of Hyenas" by BBC Earth (YouTube).
If you will forgive a bit of anthopomorphising, it really does seem that hyenas and lions might hate each other. Packs of hyenas seem to revel in (if their cackling laughter is any hint) catching and attacking lions, especially the smaller female lions caught out on the savannah alone. The larger male lions, by contrast, hunt down hyenas, and seem to specifically target the alpha female of the hyena pack, thereby aiming for maximal pack destruction. People would be hard pressed to think of two better “nemeses”: the noble, beautiful, kingly lion, and the cackling, crude, and unattractive hyena. Indeed, the folks at Disney found this story so compelling that they memorably fictionalized these true competitive interactions in the brilliant (if we speak of the first) film, “The Lion King.”
Lions and hyenas from Disney's "The Lion King" (YouTube).
Let’s suppose that packs of hyenas and lions are locked in continual and fierce interactions. We have no reason to dispute that is the case because lions and hyenas have co-occurred with each other in the same parts of Africa for roughly 3 million years. Over that span of time, neither lions nor hyenas have changed significantly in terms of their morphology, nor have they dramatically shifted their ranges relative to one another. They therefore do not demonstrate any evidence of competitive replacement and neither one is winning at some great, competitive game. There has undoubtedly been a lot of carnage over the last 3 million years—with many a hyena succumbing to a lion, and vice versa—but that competition didn’t produce continual evolutionary change or extinction. We suspect that long-term competition and hatred in the world of human behavior doesn’t usually produce a winner and a loser either; it simply produces a costly and destructive stalemate that benefits no one and should be avoided at all costs.
To top it all off, abundant evidence has been collected from the fossil record to suggest that rarely, if ever, does one species (or higher taxonomic group) drive another to extinction. Instead, classic papers by Gould and Calloway (1980) and Benton (1996)—as well as more recent works—suggest that such competitive replacements are very infrequent. The evidence for competition as a motive force of macroevolution seems more like a cultural myth than a substantiated scientific hypothesis.
If we must adopt a human caricature of evolution and competition plucked from some regal setting, it should not be a biotic Red Queen that rules over what, when, where, and how a given species goes extinct as a result of biological competition. Intensive research on the causes of both evolution and extinction has emphasized how both are largely driven by the physical environment, which is continually and whimsically shifting, only changing enough to cause speciation and extinction intermittently in evolutionary spasms of greater or lesser magnitude. Because of this, Barnosky (2001) suggested that the metaphor of a capricious Court Jester is a better fit for understanding the roles of biotic and abiotic relationships in the context of evolution and extinction. Like a court jester who is constantly moving and "working the crowd" in court, the environment is continually and seemingly whimsically shifting, driving biotic change. Key differences between the Red Queen and Court Jester hypotheses are illustrated in the figure below from Strotz et al. (2018 in Biology Letters).
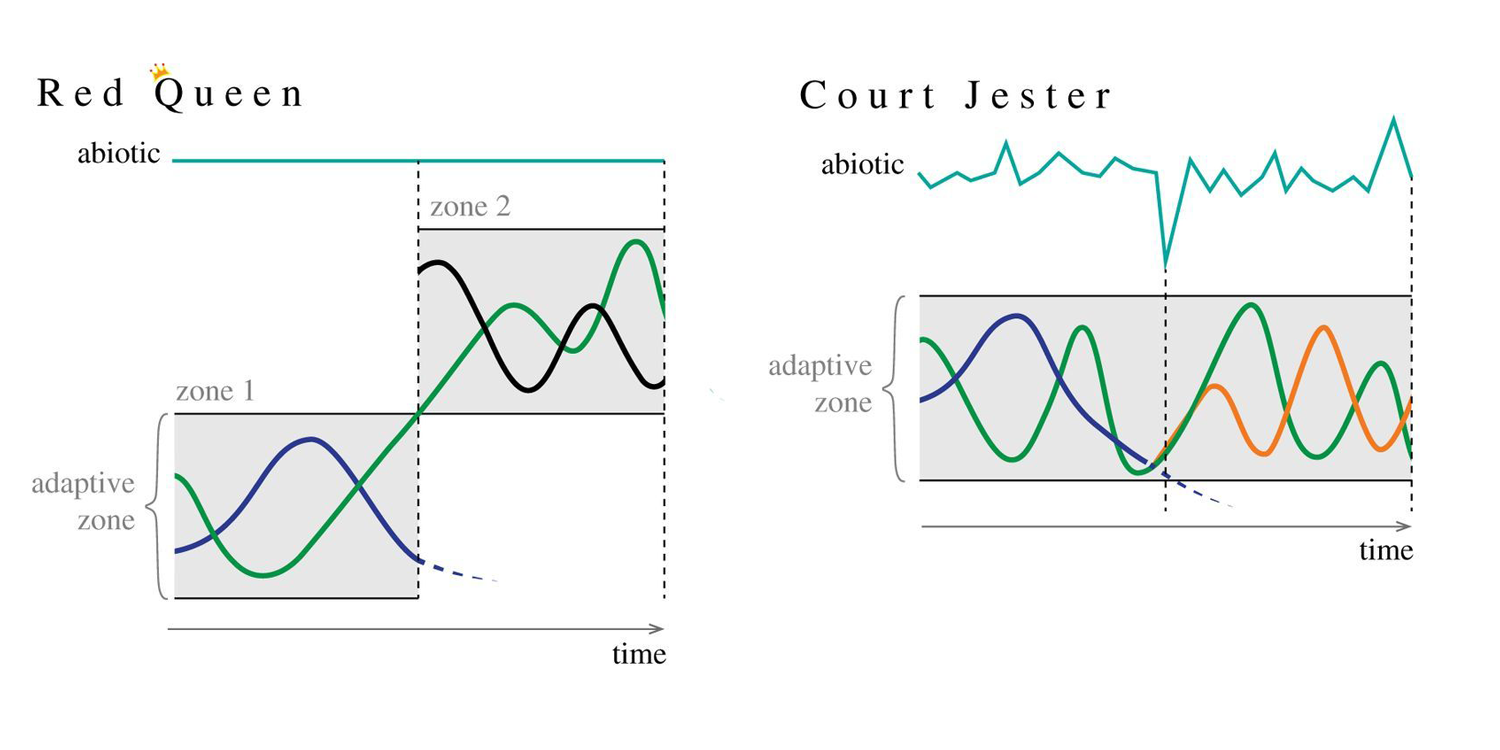
Key differences between the Red Queen and Court Jester hypotheses. Image is modified from fig. 1 in Strotz et al. (2018 in Biology Letters); the copyright for this image remains with the authors of the paper. Original caption for figure: "Evolutionary change under Red Queen hypothesis-type dynamics versus Court Jester hypothesis-type dynamics. The blue line represents the abiotic environment. Species A (green) represents a potential prey organism. Species B (purple) represents its potential predator. Species C (black) also preys on species A. Species D (orange) is a descendant taxon of species B. In both scenarios (Red Queen and Court Jester), species B goes extinct (represented by the dotted line). Under the Red Queen scenario (sensu Van Valen), the extinction of species B is due to species A shifting from adaptive zone 1 to adaptive zone 2 as it moves towards a fitness optimum and ultimately exceeds the relevant traits of species B (traits relevant to the capacity of species B to capture and consume species A). The new adaptive zone that species A now occupies contains a different predator species (species C) whose fitness is affected by the arrival of species A, as per Van Valen's zero-sum assumption. In this scenario, abiotic parameters remain unchanged and evolutionary change can still occur. In the Court Jester scenario, the extinction of species B is due to environmental changes that result in suboptimal conditions for species B. Populations within the species become isolated from one another, population sizes decrease, and almost all of the component populations die off, such that species B goes extinct; however, in one case an isolated population of species B diverges, survives and becomes a new species (species D). No changes in the adaptive zone of species A occur in this scenario, nor did they the cause the extinction of species B. Both hypotheses have different evolutionary and spatial scales. The RQH operates across individual populations on small spatial and short temporal scales, leading to differential survival of populations within communities. By contrast, the Court Jester hypothesis is tied to large-scale shifts in the physical environment, which would affect multifarious populations from species in different clades, with each population potentially responding individualistically to the perturbation."
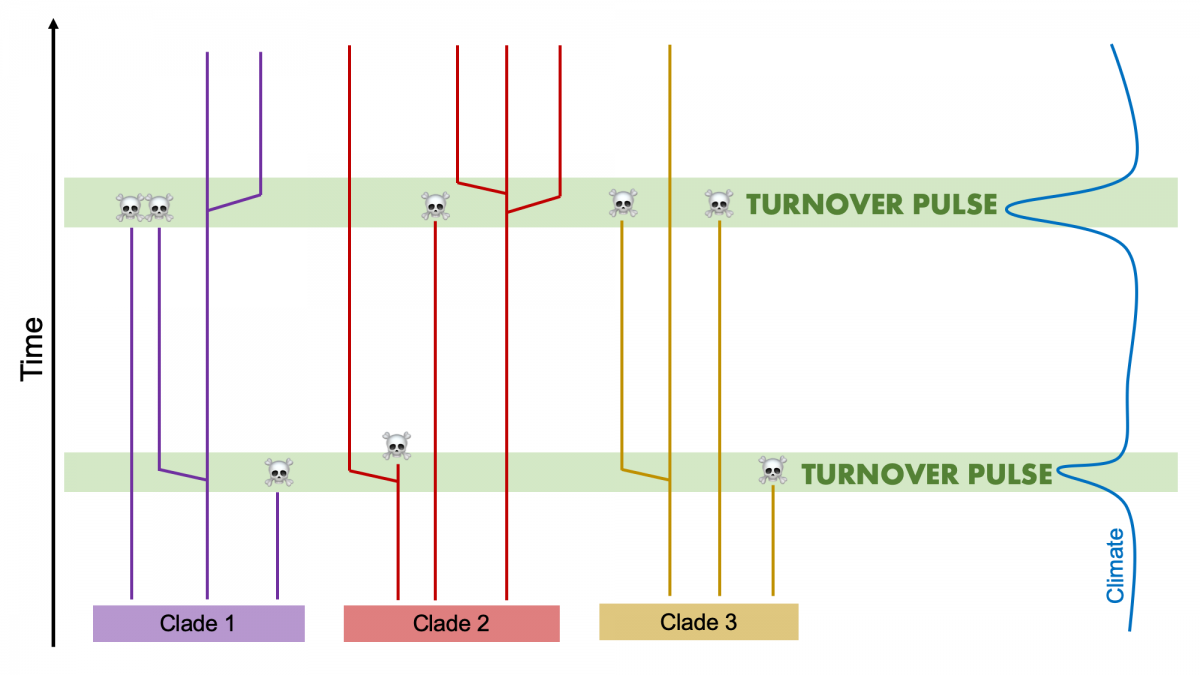
The Turnover-Pulse Hypothesis
Elisabeth Vrba (Vrba, 1980, 1985, 1993) formulated a key macroevolutionary concept called the Turnover-Pulse hypothesis that emphasized the prominent role that environmental change plays in motivating evolution. This hypothesis, which is a type of Court Jester model, took the concept of punctuated equilibria and extended it from the purview of single lineages evolving one at a time to the evolution of multi-species assemblages. The Turnover-Pulse hypothesis posits that populations of species first respond to climate change via migration. If the changing climate produces an improvement in their preferred habitat, they will expand their range. However, if it leads to a deterioration in conditions, ranges of populations will become restricted to narrow, isolated refugia and they will be more likely to disappear. This situation reflects what happened to tropical mammals during the cooling phase of the most recent Ice Ages.
During intervals of climate change that lead towards less suitable conditions, groups of species that occur in a region are expected to first show contractions in geographic range and then "turnovers," with increased rates of extinction and also coeval "pulses" of speciation. This is because if species are able to persist in isolated refugia, they are more likely to become fixed for certain key characters such that they rapidly diverge via the process of allopatric speciation (see earlier section of this chapter). In a phylogenetic context, then, a turnover-pulse event would show coincident patterns of extinction and speciation during short intervals of time across a variety of different clades.
Subsequent work has shown that turnover pulses not only apply to Vrba’s paradigm example of tropical mammals from the Neogene of South America and Africa, but also to fossil invertebrates from the Paleozoic of North America, where they have been discussed at times under the rubric of Coordinated Stasis (Brett and Baird, 1995; Morris et al., 1995).
Vrba’s work leads to the important realization that not only do species show long periods of stasis followed by relatively short periods of rapid change, but so do entire assemblages of species belonging to different clades. Importantly, her hypothesis specifies that climate change plays a crucial role in driving these patterns. Furthermore, Turnover Pulse provides an explanation for one of the fundamental patterns observed in the fossil record: the fossil record and geological time can be split up into different intervals or biostratigraphic stages that are bracketed at the top by the relatively sudden disappearance of many fossil species and at the bottom with again the relatively sudden emergence of a plethora of new species. It was a key insight by Vrba to recognize that much of the macroevolution that goes on in the history of life seems to occur during these profound turnovers and pulses associated with times of major environmental change, with fewer changes occurring during periods of environmental stability.
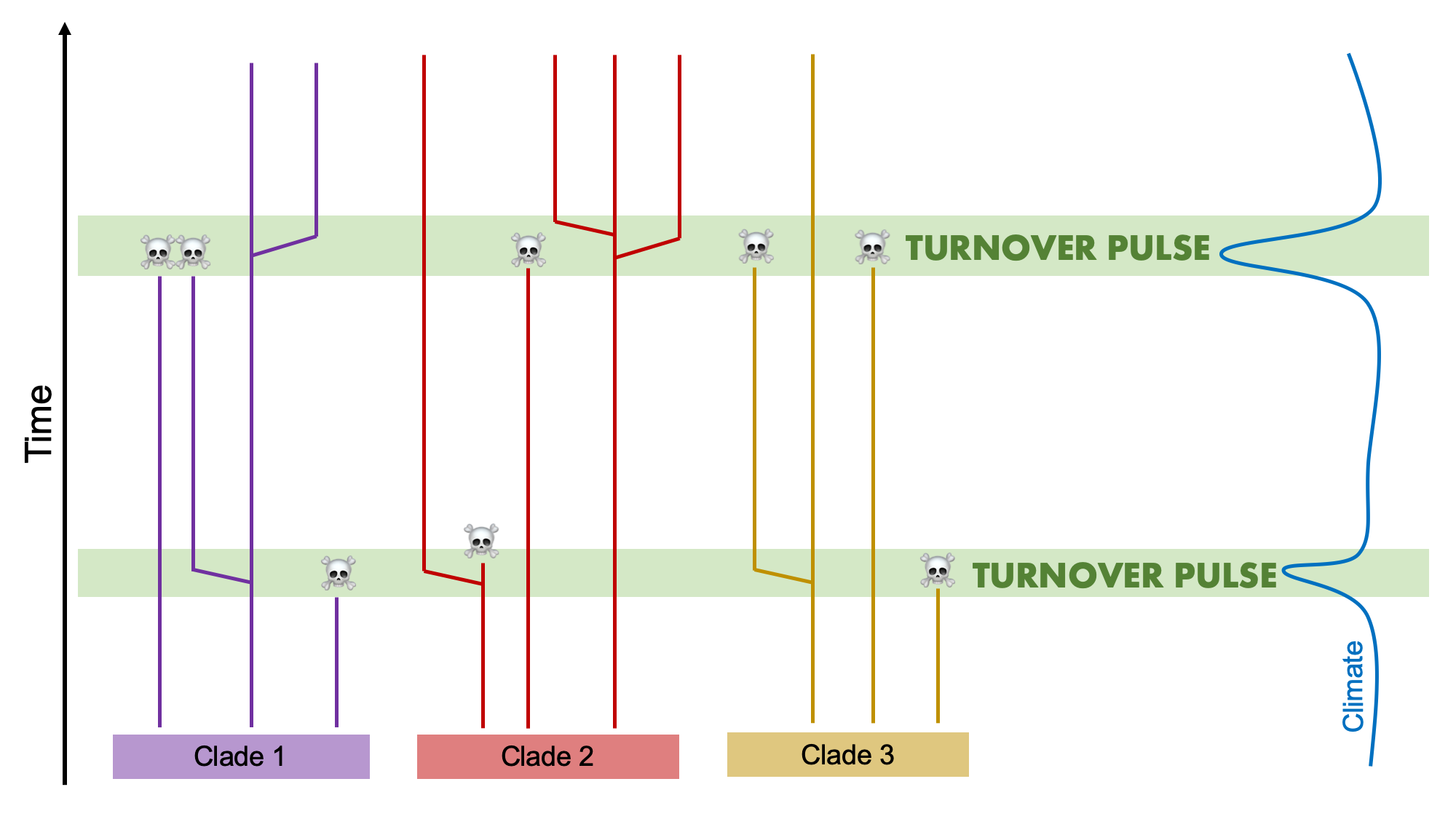
Illustration of Vrba's Turnover-Pulse hypothesis, which predicts that during intervals of climate change, species belonging to different clades will show nearly coincident patterns of extinction and speciation. Image by Jonathan R. Hendricks is based on fig. 4 in Vrba (1993).

Paleobiologist Elisabeth Vrba. Image by "Gerbil" (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license).
Conclusions
Undoubtedly, when it comes to phenomena like competition and climate change and their effects on macroevolution, both play some role. The key challenge is to determine which predominates in a given system. New analytical techniques like ecological niche modeling (see prior section) make it possible to consider each aspect in concert and produce quantitative estimates of their effects. These niche modeling studies have also reiterated the results from the aforementioned analyses of the fossil record and emphasize that the physical environment is the leading pacemaker of evolutionary change over long time scales, while competition plays a more subsidiary role.
References and further reading
Antell, G., Kiesling, W., Aberhan, M., and Saupe, E. E. 2019. Ecological release is undetectable in the sea after global catastrophes throughout Earth history. Current Biology. In press.
Barnosky, A. 2001. Distinguishing the effects of the Red Queen and the Court Jester on Miocene mammal evolution in the northern Rocky Mountains. Journal of Vertebrate Paleontology 21: 172-185.
Benton, M. J. 1996. Testing the roles of competition and expansion in tetrapod evolution. Proceedings of the Royal Society, Series B. 263: 641-646.
Benton, M. J. 2009. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323: 728-732.
Brett, C. E., and G. C. Baird. 1995. Coordinated stasis and evolutionary ecology of Silurian to Middle Devonian faunas in the Appalachian Basin. Pp. 285-315 in: Erwin, D. H., and R. L. Anstey, New Approaches to Speciation in the Fossil Record, Columbia University Press, New York.
Brockhurst, M. A., Chapman, T., King, K. C., Mank, J. E., Paterson, S., and Hurst, G. D. 2014. Running with the Red Queen: the role of biotic conflicts in evolution. Proceedings of the Royal Society, Series B. 281:20141382.
Darwin, C. 1859. On the Origin of Species by Means of Natural Selection (facsimile 1st edition). Harvard University Press, Cambridge, Mass.
Eldredge, N. 1989. Macroevolutionary Dynamics, Englewood Cliffs, NJ.
Finnegan, S., J. L. Payne, and S. C. Wang. 2008. The Red Queen revisisted: reevaluating the age selectivity of Phanerozoic marine genus extinctions. Paleobiology 34(3): 318-341.
Foin, T. C., J. W. Valentine, and F. J. Ayala. 1975. Extinction of taxa and Van Valen's law. Nature 257: 514-515.
Gould, S. J., and Calloway, C. B. 1980. Clams and brachiopods – ships that pass in the night. Paleobiology 6: 383-396.
Kemper, S. 2008. Who's laughing now? Smithsonian Magazine: https://www.smithsonianmag.com/science-nature/whos-laughing-now-38529396/.
McCune, A. R. 1982. On the fallacy of constant extinction rates. Evolution 39:610-614.
Morris, P. J, Ivany, L. C., Schopf, K. M., and Brett, C. E. 1995. The challenge of paleoecological stasis: reassessing sources of evolutionary stability. Proceedings of the National Academy of Sciences, U.S.A. 92: 11269-11273.
Myers, C. E., and Lieberman, B. S. 2011. Sharks that pass in the night: Using GIS to investigate competition in the Cretaceous Western Interior Seaway. Proceedings of the Royal Society, Series B. 278:681-689.
Myers, C. E., and Saupe, E. E. 2013. A macroevolutionary expansion of the modern synthesis and the importance of extrinsic abiotic factors. Palaeontology 56:1179-1198.
Raup, D. M. 1975. Taxonomic survivorship curves and Van Valen's law. Paleobiology 1(1): 82-96.
Ross, R. M., and Allmon, W. D. 1990. The Causes of Evolution: A Paleontological Perspective. University of Chicago Press, Chicago, Ill.
Simpson, G. G. 1944. Tempo and Mode in Evolution. Columbia University Press, New York, NY.
Saupe, E. E., Farnsworth, A., Sagoo, N., Lunt, D., and Field, D. J. 2019. Climatic shifts drove major contractions in avian latitudinal distributions throughout the Cenozoic. Proceedings of the National Academy of Sciences, U.S.A. 116: 12895-12900.
Strotz, L. C., Simões, M., Girard, M., Breitkreuz, L., Kimmig, J., and Lieberman, B. S. 2018. Getting somewhere with the Red Queen: Chasing a Biologically Relevant Definition. Biology Letters 14: 20170734, 1-7. http://dx.doi.org/10.1098/rsbl.2017.0734
Van Valen, L. 1973. A new evolutionary law. Evolutionary Theory 1: 1-30.
Vermeij, G. J. 1994. The evolutionary interaction among species: selection, escalation, and coevolution. Annual Review of Ecology and Systematics 25: 219-236.
Vermeij, G. J. 2013. On escalation. Annual Review of Earth and Planetary Sciences 41: 1-19.
Vrba, E. S. 1980. Evolution, species and fossils: how does life evolve? South African Journal of Science 76: 61-84.
Vrba, E. S. 1985. Environment and evolution: alternative causes of the temporal distribution of evolutionary events. South African Journal of Science 81: 229-236.
Vrba, E. S. 1993. Turnover-pulses, the Red Queen, and related topics. American Journal of Science 293A: 418-452.
Content usage
Usage of text and images created for DEAL: Text on this page was written by Bruce S. Lieberman and Jonathan R. Hendricks. Original written content created by Bruce S. Lieberman and Jonathan R. Hendricks for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images created by Jonathan R. Hendricks are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.