Chapter contents:
Evolution and the Fossil Record
– 1. Natural selection
– 2. Species and species concepts
– 3. Speciation ←
– 4. Punctuated equilibria and stasis
–– 4.1 Videos about punctuated equilibrium and stasis
– 5. Macroevolution
–– 5.1 Hierarchies
–– 5.2 Species selection
–– 5.3 Abiotic vs. biotic causes of macroevolution
–– 5.4 Evolutionary radiations
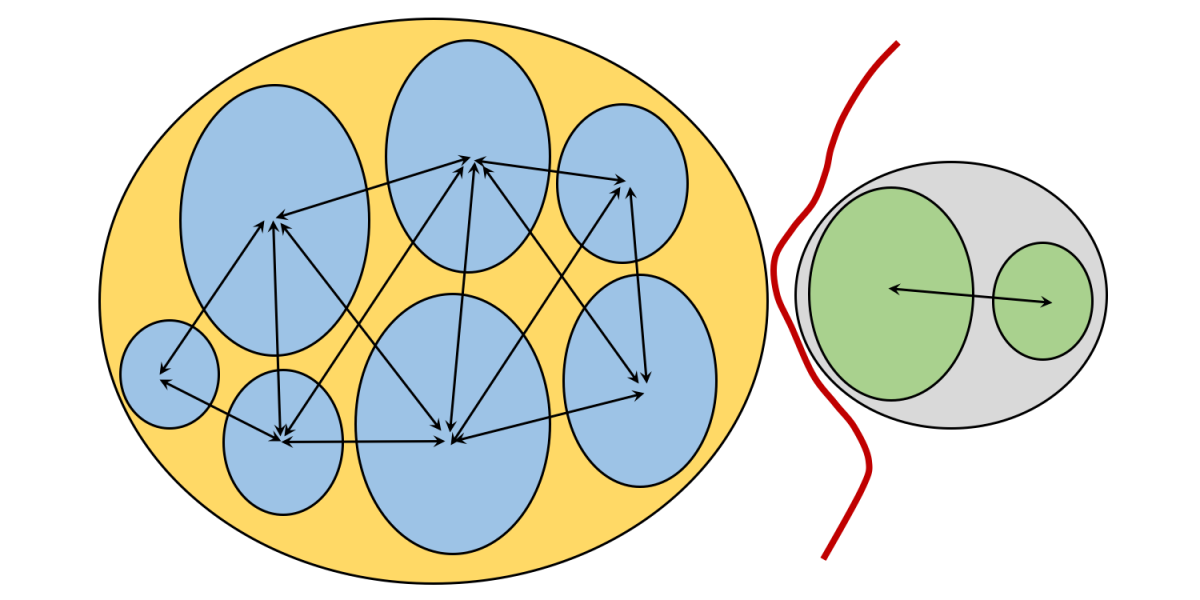
Image above: a simplified model for the process of allopatric speciation.
Thus far in this chapter, you have been introduced to the biological nature of species (Sec. 2) and have learned how the process of natural selection (Sec. 1) results in organisms being well adapted to the environments in which they live. We now want to consider the important matter of the "origin of species," or speciation. Speciation is the evolutionary process (or processes) that cause new species (i.e., descendant taxa) to evolve from existing species (i.e., ancestral taxa). While no credible evolutionary biologist or paleontologist questions the fact of evolution (i.e., that species have evolved from prior forms), scientists do actively debate the processes that are most responsible for the evolutionary patterns observed in nature. Studies have shown that there are multiple natural processes that drive (and have driven) speciation. Based on extensive surveys of modern and fossil species, however, the phenomenon of allopatric speciation has proven to be the most important process, accounting for an estimated 90-95% of speciation events. We will, therefore, focus here mostly on this mode of speciation.
Allopatric speciation
Allopatric speciation is the idea--often attributed in part to Harvard ornithologist Ernst Mayr--that new species originate when small, localized populations within an established species become geographically isolated from all of the other populations within the species due to the formation of physical barriers to gene flow. Physical barriers include the formation of mountain belts, rivers, seaways, and more.
Imagine a widely ranging species composed of numerous populations of individual organisms. Males and females most often reproduce with individuals from within their own population. Occasionally, however, individuals move between adjacent population groups and mate with members of those other populations, establishing genetic connections between the different populations. All are members of the same species, so this is not a problem and these genetic connections help to maintain the distinctiveness of the species across its wide range. This scenario is illustrated in the simple diagram below.
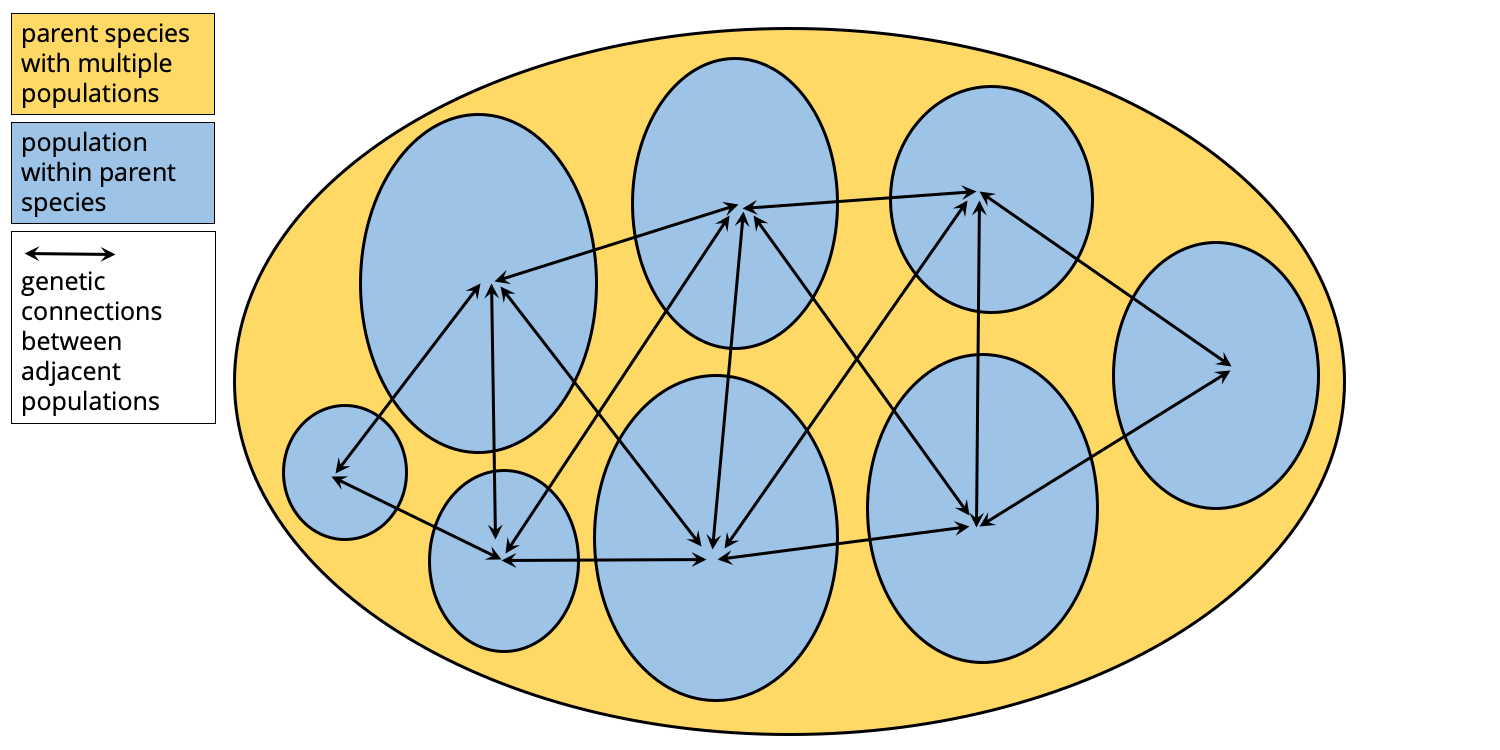
A wide-ranging species composed of numerous populations of reproducing individuals. Individuals also occasionally mate with members of adjacent populations, establishing genetic connections between populations. Image by Jonathan R. Hendricks.
Now, suppose that a barrier formed that physically isolated one population from all of the others. This physical barrier could, for example, be a newly formed tall mountain range, a wide river, or a shallow seaway. Note that barriers to some kinds of organisms are bridges for other. For example, a newly formed river could divide populations of land-dwelling animals, but form new conduits for aquatic species.
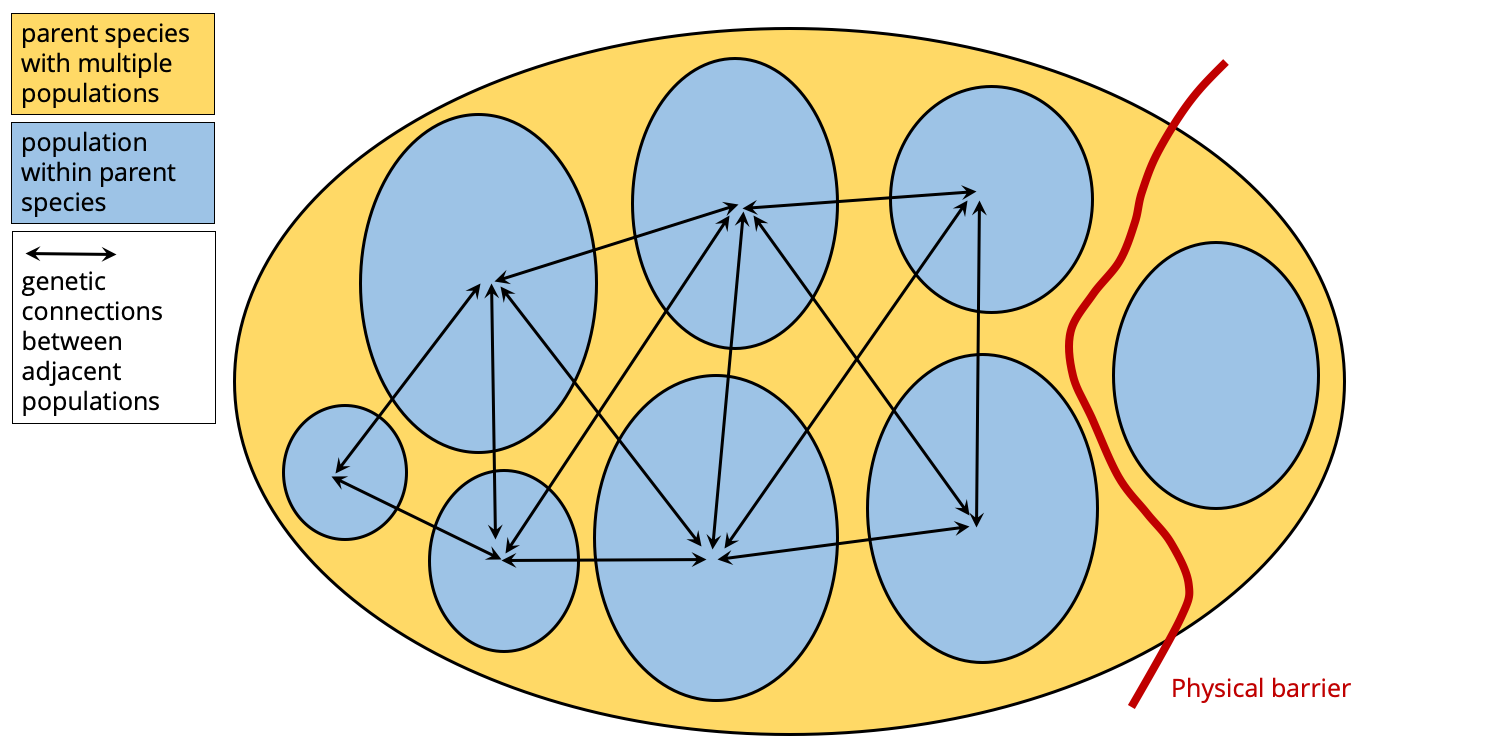
A physical barrier forms, genetically isolating one population from its parent species. Image by Jonathan R. Hendricks.
One of the most important physical barriers to form in the recent geological past was the Isthmus of Panama. The Isthmus finished forming (due to tectonic processes) approximately 3 million years ago. This created a natural land bridge that connected North America with South America, initiating an event called the "Great American Biotic Interchange" (or, GABI).
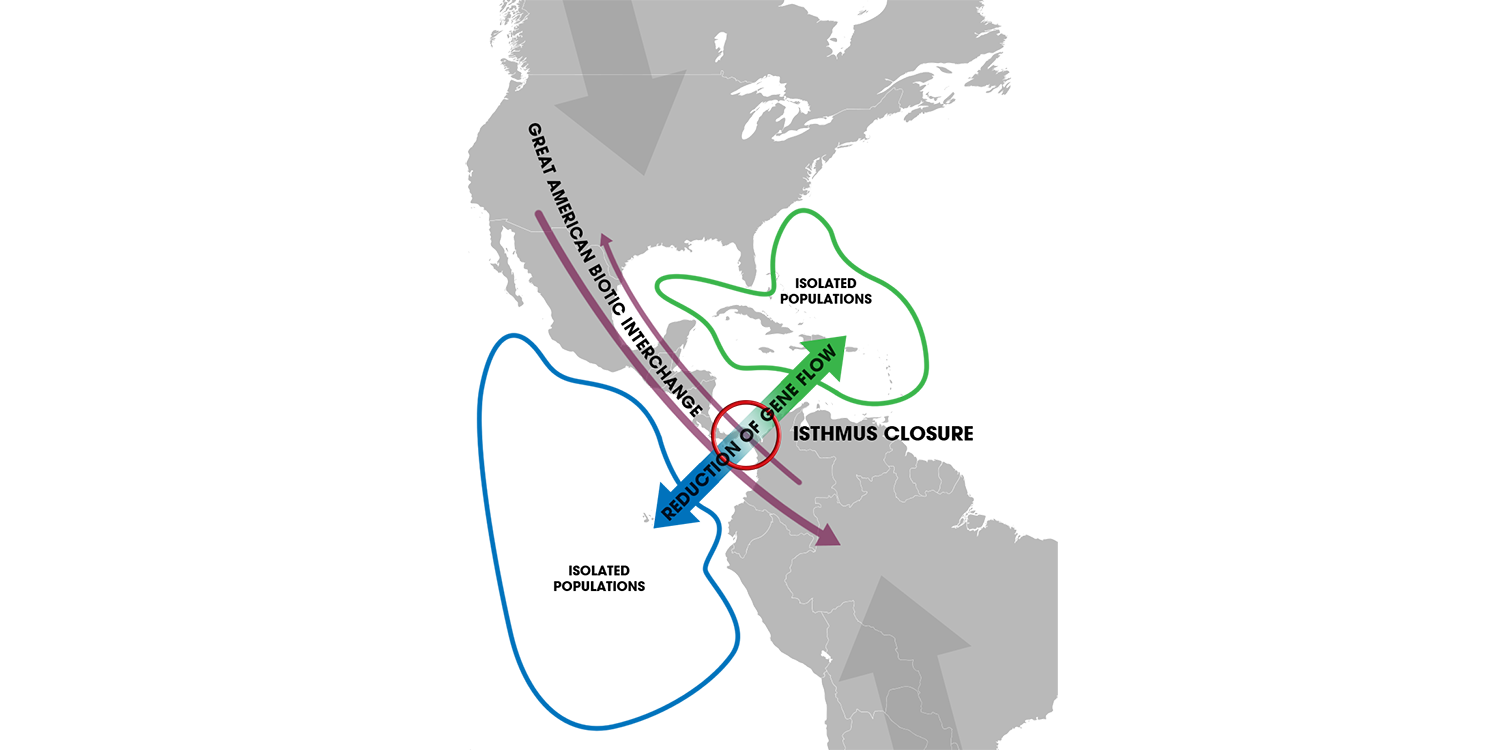
The formation of the Isthmus of Panama about 3 million years ago created a physical barrier that separated the eastern Pacific from the western Atlantic. This barrier for marine life became, however, a land bridge for terrestrial species. Image by Andrew Z. Colvin (Wikimedia Commons; Creative Commons Attribution-Share Alike 4.0 International license).
During the the GABI, many North American animal lineages dispersed into South America, while many South American animal groups moved into North America (including giant ground slots, glyptodonts, and terror birds). An impassible barrier was created, however, for marine animals in this region.
Source: "What happens when continents collide" by Juan D. Carrillo for TED-Ed (YouTube).
When small populations become physically isolated, new and favorable mutations may spread very quickly by natural selection or random effects such as genetic drift. This can lead to rapid evolutionary divergence (at least in a geological context). Over hundreds or thousands of generations, members of the isolated population may become very different from members of the parent population in terms of their appearances, behaviors, as well as in their underlying genetics.
If individuals from the two populations happen to come back into contact with each other and mate, their offspring may not be viable or the hybrids that they produce may be infertile. Alternatively, the two individuals may no longer recognize each other as belonging to the same species. Indeed, some species have particular visual cues that they use to recognize members of their species (examples include the horns of antelopes and the feather coloring of many birds). Other cues are behavioral (for example, birds often use songs and/or dances to recognize members of their own species). We call these morphological or behavioral cues "mate recognition systems" and the birds of paradise shown in the video below provide great examples of both.
Source: "Bird Of Paradise Courtship Spectacle" by BBC Earth / Planet Earth (YouTube).
Whether individuals from the parent and isolated populations are unable to successfully mate, or don't even try, they are effectively different species at this point and speciation has occurred. The new species (the one that began as an isolated population) is sometimes referred to as the "daughter species."
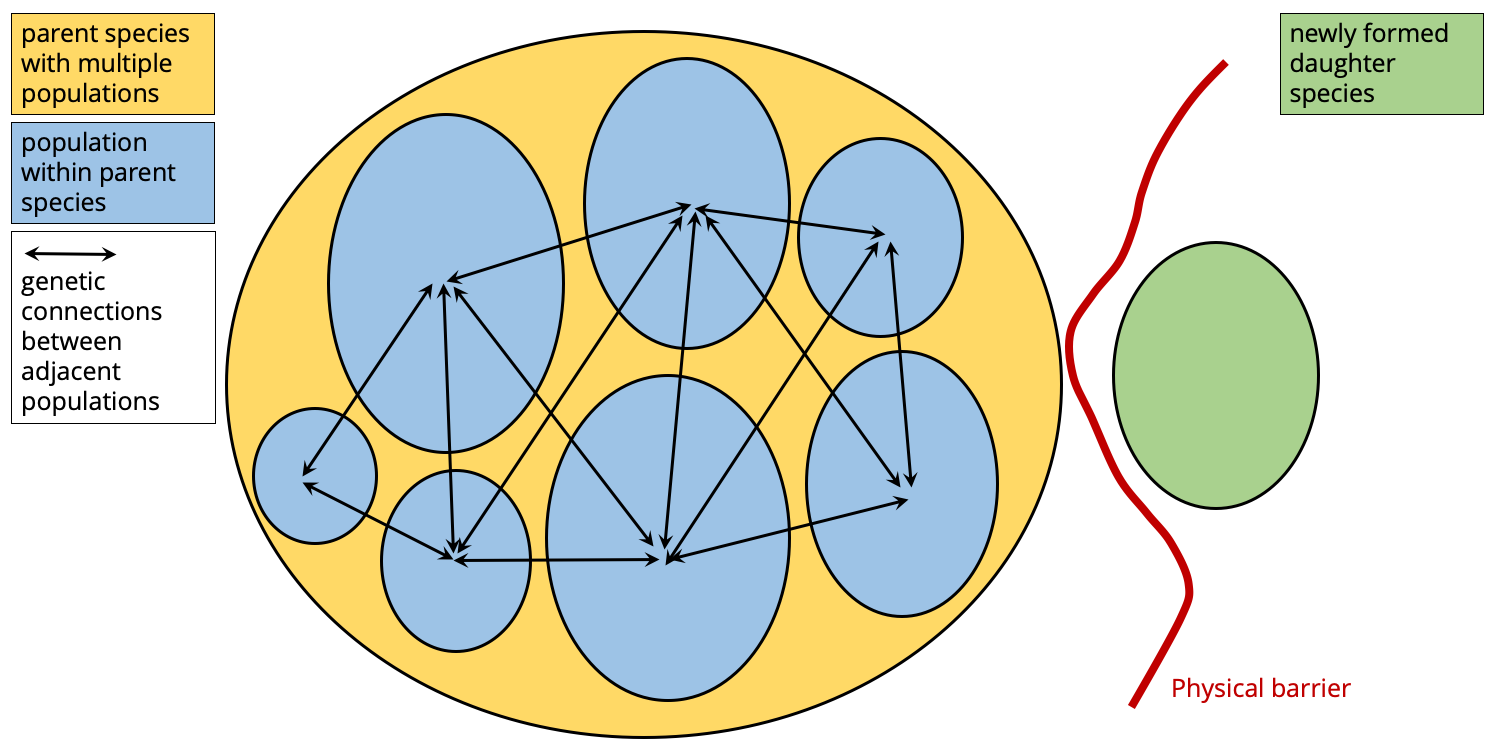
Small, isolated populations may genetically diverge very quickly. If enough time passes, speciation may occur, resulting in the formation of a new daughter species. Image by Jonathan R. Hendricks.
If the new daughter species is successful, its range may begin to expand. As this happens, new populations will form within the daughter species. It is possible that these populations may even expand back into the range of the parent species if the physical barrier fails over time. This is when the biological boundaries between the parent and daughter species will truly be tested.
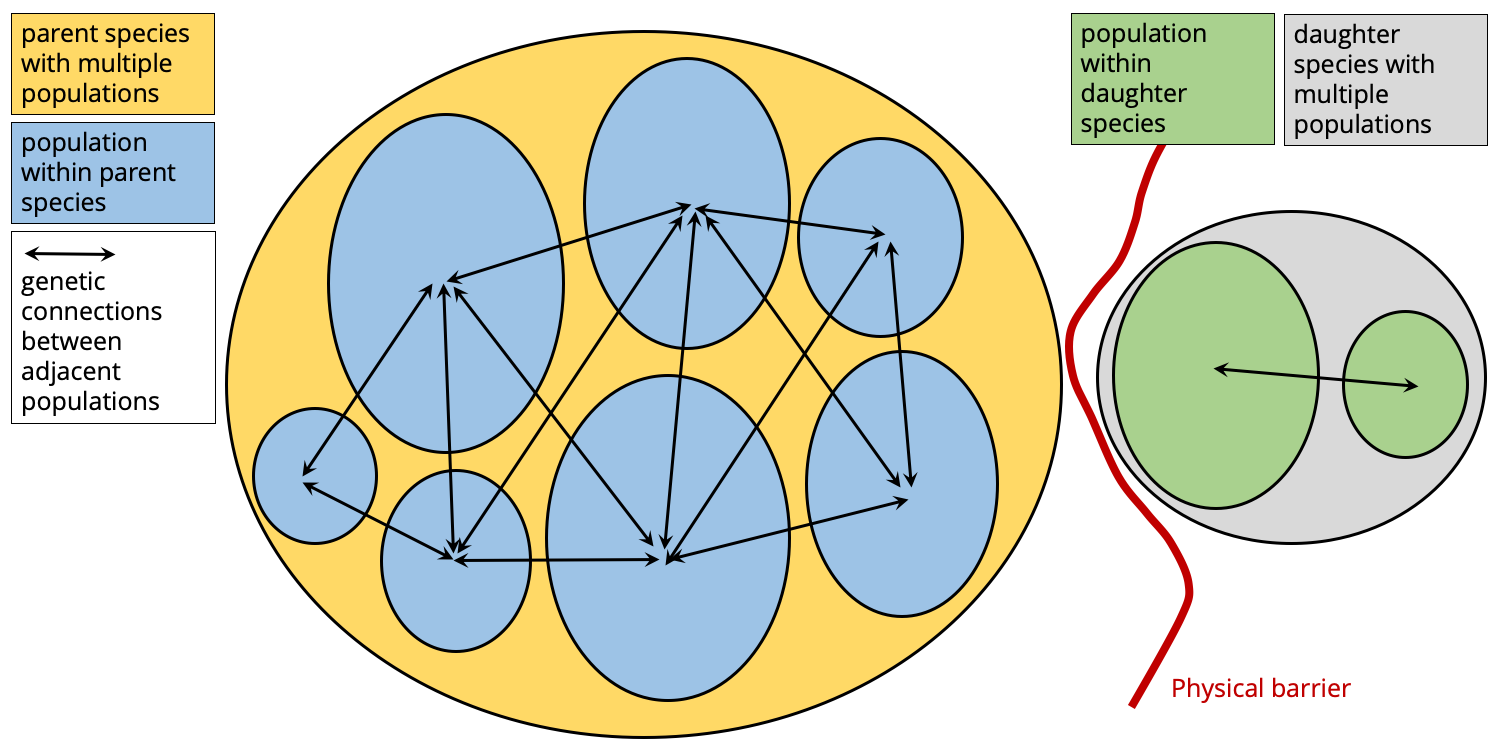
As the range of the daughter species expands, new populations will form. Image by Jonathan R. Hendricks.
In the scenario above, a hypothetical landscape was fragmented by a newly formed physical barrier that caused the isolation of one population. This is referred to as vicariance speciation. Additional models exist, however, within the allopatry framework. One in particular is peripatric speciation. In peripatric speciation, a population becomes isolated by crossing an existing physical barrier, often due to chance events. For example, we think that much of the native endemic biota of the remote Hawaiian islands originally arrived by chance from far away continents. The video below first details such an example of peripatric speciation, then follows with an example of vicariance speciation.
Source: "Speciation: An Illustrated Introduction" by the the Cornell Lab of Ornithology (YouTube).
Fuzzy boundaries between closely related species
In some cases, the distinctions between species boundaries may not entirely hold up to the definitions that we have imposed upon them. An example comes from the history of our own species, Homo sapiens.
Today, Homo sapiens is the only living species of humans. Not so long ago (geologically speaking), we co-existed on Earth with several other species of humans, one of which was Homo neanderthalensis (or, the Neanderthal). While very similar to our species overall, Neanderthals nevertheless had morphological features that differentiate them from us, perhaps most notably their much more robust brow ridge. There is evidence that, like us, Neanderthals were socially complex. For example, we have evidence that they created tools and art, buried their dead, and looked after injured group members. Their brains were also larger than ours.
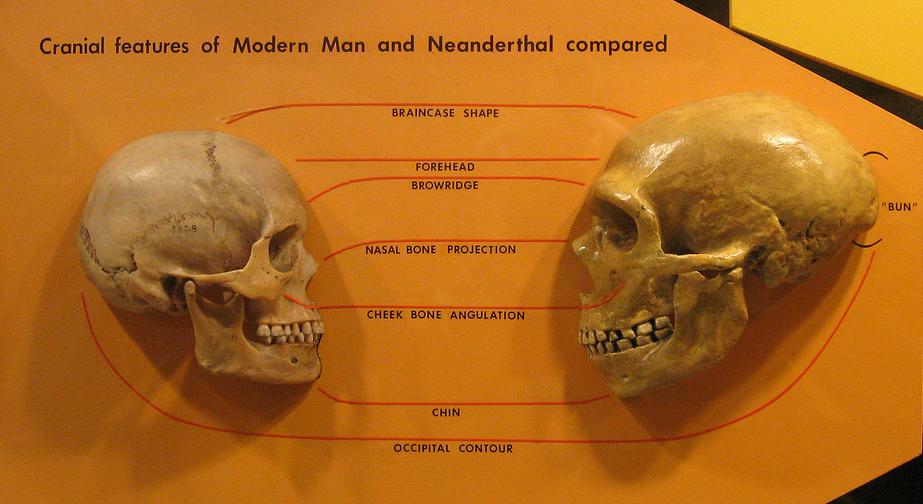
A comparison of the skulls of Homo sapiens and Homo neanderthalensis. Image by "hairymuseummatt" (Wikimedia Commons; Creative Commons Attribution-Share Alike 2.0 Generic license).
Interactive 3D model of a Neanderthal skull by "seminaronscience" at the American Museum of Natural History (Sketchfab).

Reconstructions of the faces of ancient Homo sapiens (left) and Homo neanderthalensis (right). Image by "The Nature Box" (Wikimedia Commons; Creative Commons Attribution-Share Alike 4.0 International license).
Some members of our species, Homo sapiens, left Africa about 60,000 years ago and moved northward. When they arrived in Europe around 40,000 years ago (or perhaps somewhat earlier), they encountered Neanderthals. Our two species co-existed in Europe for at least several thousand years. We do not know what, if any, role(s) modern humans played in their demise. Did we kill them, either intentionally, or unintentionally through the diseases that we brought with us? Or did climate change (cooling) doom the Neanderthals? What we do know is that Homo sapiens and Neanderthals interbred.
Source: "Neanderthals 101" by National Geographic (YouTube).
Neanderthal fossils are geologically very, very young and some have been buried in conditions favorable to the preservation of DNA (there is no good evidence for preservation of DNA in multicellular fossils older than around a million years). Because of this, scientists have been able to decode the entire genome, or genetic code, of the Neanderthal. Comparison of the genomes of modern humans and Neanderthals show them to be 99.5% identical. Perhaps more surprising, however, has been the discovery that most humans have about 2.5% Neanderthal DNA in their genomes, the exception being people of sub-Saharan African ancestry, who have none. In fact, it is even possible to determine the amount of Neanderthal DNA that is present in your genome through commercial genetic testing services such as 23andme.com.
The fact that Homo sapiens and Neanderthals interbred is important for our consideration of species boundaries and speciation events. We know that for most of their respective histories (spanning tens of thousands of years), Homo sapiens and Neanderthals were geographically isolated from each other. If they belonged to different species, how were they able to successfully reproduce? The answer is that while numerous differences had accumulated between the two long-isolated species, complete reproductive isolation had not yet occurred. This likely stems from the fact that both Homo sapiens and Neanderthals apparently diverged from a shared common ancestor (Homo heidelbergensis) only around 400,000 years ago.
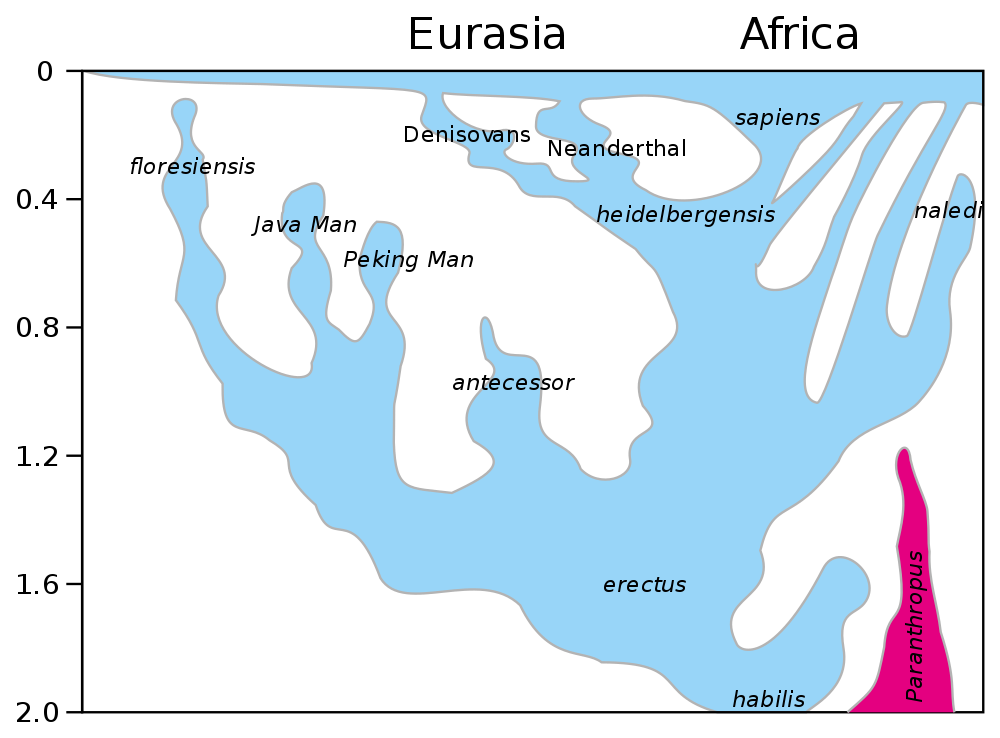
The evolutionary history of the genus Homo (humans). Note that Neanderthals and Homo sapiens both apparently evolved from Homo heidelbergensis about 400,000 years ago. Image by "Conquistador" and "Dbachmann" (Wikimedia Commons; Creative Commons Attribution-Share Alike 4.0 International license) is based on figure in Stringer (2012).
The interbreeding events also seem to have been both rare and short lived, as Neanderthals went extinct soon after encountering Homo sapiens around 40,000 years ago. If interbreeding had persisted for a much longer period of time, it is conceivable that the two species might have merged into one. But, this did not occur. This close-to-home example instead presents an interesting case of "reproductive leakage" between two evolutionary species and we think cases like this are fairly rare in nature and happen less than 2% of the time (if it is more common than this, then the two species will just fuse back into one upon the re-establishment of contact). A similar phenomenon to what we have just discussed in Homo sapiens and Neanderthals also has been shown to occur in other species.
When it comes to reproductive preferences, especially between closely related species that have not yet fully diverged, we sometimes observe that they can fit the pattern discussed in the systematics chapter regarding homologies and the existence of shared derived (synapomorphies) and shared primitive characters (plesiomorphies). Males of some species of the freshwater fish genus Xiphophorus have an elongated tail fin, giving them the common name "swordtails" (females never have this feature). Presence of such a "swordtail" is considered the primitive condition (meaning it was possessed by the shared common ancestor of all species of Xiphophorus). Males of other species of Xiphophorus lack this feature, which is considered the derived condition (these species are called "platyfish").

The green swordtail, Xiphophorus helleri. Image by "Usien" (Wikimedia Commons; Creative Commons CC0 1.0 Universal Pubic Domain Dedication).

A male of the variatus platy (Xiphophorus variatus), which lacks a sword tail. Image by "Marrabbio2" (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license).
The species of Xiphophorus with long tails ("swordtails") and those without ("platys") are currently geographically isolated in different rivers in Central America and Mexico. Scientists have done experiments where they have literally glued a swordtail back on to males of the swordless "platy" species. What do the female platys think of these "enhanced" males? They love them! When given the choice, female platys prefer individuals that have an artificial swordtail over males that lack this feature. Thus, the preference for the primitive state (presence of a swordtail) is retained.
What should we expect in the long term if a swordless platy species comes back into contact in the wild with a swordtail species? If they can still interbreed, then eventually the swordless form will be swamped out because female platys prefer this condition (an example of sexual selection). What is fascinating about this is that it means that there are times when we can actually watch the phenomenon of speciation in action. New species don’t generally appear instantaneously from their ancestors. Instead, the development of genetic boundaries is likely often protracted over many, many generations. Does this suggest that species don't exist and are not real? Not at all. Instead, this is just a great window into how speciation can take many thousands of years to happen.
The nature of species, revisited
You have already learned about the nature of species earlier in this chapter. Let's consider them again in the context of what you have just learned about speciation. Many scientists have come to think of species as representing something akin to organisms, but at a higher level: individual organisms are born, live, and die, just as species originate, persist, then go extinct. The timing of when life begins for an individual organism, of course, is not entirely obvious. For humans, some consider it to be at the time of conception, but for others it is when a developing fetus develops an independent heartbeat. Still others consider life beginning at the time of birth when the umbilical cord is cut, or even later when the child’s college tuition is paid off.
Consider also the process of "birth" in less familiar, single-celled organisms like Paramecium, which sometimes asexually reproduce by dividing in two.
Source: "Paramecium tutorial HD" by "mantismundi" (YouTube). Note video of dividing Paramecium at 3:22.
During the process of division, it is unclear when the newly budding organism becomes truly separate from its "parent." Is it when the nucleus of the newly budding "offspring" first appears? Is it when some percentage of the budding offspring has formed (e.g., 50%?). Or, is it—at the other extreme—when the two Paramecium are no longer surrounded by a single membrane? Even in this final instance, after a single Paramecium fully splits into two Paramecium, the new Paramecium often sticks around in the vicinity of the old Paramecium for about 5 minutes before it moves on. Are these individuals not truly independent until the new one departs the scene? This is a question for which we have no concrete answer. Defining when life begins for an organism is akin to defining when a new species has become fully independent from its ancestor: it is somewhat fuzzy and can often only be defined subjectively. To us, the distinction between a newly evolving species and its ancestral species is much like the distinction between night and day: the differences between midnight and noon are obvious, but the differences are less obvious between dusk and dawn. It is for similar reasons that we say that species exist, even though it can sometimes be tricky to precisely identify the boundaries that define them (e.g., consider the case of the Neanderthals, reviewed above).
Just as we can make an analogy between the birth of an individual organism (however you want to define it) and the process of speciation, we can also consider the nature of species once they are fully established. Some scientists view established species as analogous to individual organisms. Further, just as individual organisms are affected by the process of natural selection, an analogous process that operates at a higher macroevolutionary level might involve selection of entire species. This phenomenon, which is known as species selection, is discussed later in this chapter.
Part of the legacy of why species and species concepts have been so confusing has to do with Darwin and his notion of species. The basic presumption of creationists who were operating in the first part of the 19th century, before evolution started to become accepted in scientific circles, was that all species had been independently created by God and were immutable. Darwin did a clever thing to get around this in his book On the Origin of Species.

Title page of the first edition (1859) of Darwin's On the Origin of Species (public domain).
Darwin knew that many scientists, as well as the public, might be resistant to the notion that species could change or evolve through time. By contrast, they would not doubt that within-species change could and did occur (many modern Creationists accept this as well). Because of this, Darwin argued that really there was no difference between a species, a sub-species, or a variety. Darwin explained that there was a complete range or swath of variation extending from organisms up through populations to species, and beyond. If there really was no difference between a population and a species, and if people accepted that populations could change through time, then he had cut the Gordian knot and species could now be viewed as also being subject to changes over time. For this reason, it is somewhat ironic that Darwin titled his book the On the Origin of Species, given that he basically said in this book that species don’t even really exist. This view was hugely influential and was part of the reason why there was so much subsequent confusion and debate about the nature of species.
Despite its achievement in convincing most scientists that evolution was a fact of nature—as well as providing a mechanism for explaining adaptations and within-species changes over time (natural selection)—Darwin's opus says little about how species originate. It also presented the view that species must gradually change during the interval between speciation and extinction (that is, they must adapt to changing conditions, or perish). This perspective was challenged in the early 1970's, however, by the introduction of the idea of punctuated equilibrium, a concept which will be explained in the next section.
References and further reading
Allmon, W. D., and M. M. Yacobucci (eds.). 2016. Species and Speciation in the Fossil Record. University of Chicago Press, Chicago, 384 pp.
Darwin, C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London, 513 pp.
Erwin, D. H., and R. L. Anstey (eds.). 1995. New approaches to speciation in the fossil record. Columbia University Press, New York, 342 pp.
Harris, K., and R. Nielsen. 2017. Q&A: Where did the Neanderthals go? BMC Biology 15:73.
Mayer, E. 1942. Systematics and the origin of species from the viewpoint of a zoologist. Cambridge, Harvard University Press, 334 pp. [Reprinted 1999.]
Ottenburghs, J., P. van Hooft, S. E. van Wieren, R. C. Ydenberg, and H. H. T. Prins. 2016. Hybridization in geese: a review. Frontiers in Zoology 2016 13: 20.
Stringer, C. 2012. What makes a modern human. Nature 485: 33-35.
Content usage
Usage of text and images created for DEAL: Text on this page was written by Bruce S. Lieberman and Jonathan R. Hendricks. Original written content created by Bruce S. Lieberman and Jonathan R. Hendricks for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images created by Jonathan R. Hendricks are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.